Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inhibition of TRPM7 blocks MRTF/SRF-dependent transcriptional and tumorigenic activity.
Oncogene ( IF 6.9 ) Pub Date : 2019-12-16 , DOI: 10.1038/s41388-019-1140-8 Sandra Voringer 1 , Laura Schreyer 1, 2 , Wiebke Nadolni 1 , Melanie A Meier 1, 2 , Katharina Woerther 1 , Constanze Mittermeier 1 , Silvia Ferioli 1 , Stephan Singer 3 , Kerstin Holzer 3 , Susanna Zierler 1 , Vladimir Chubanov 1 , Bernhard Liebl 4 , Thomas Gudermann 1 , Susanne Muehlich 1, 2
Oncogene ( IF 6.9 ) Pub Date : 2019-12-16 , DOI: 10.1038/s41388-019-1140-8 Sandra Voringer 1 , Laura Schreyer 1, 2 , Wiebke Nadolni 1 , Melanie A Meier 1, 2 , Katharina Woerther 1 , Constanze Mittermeier 1 , Silvia Ferioli 1 , Stephan Singer 3 , Kerstin Holzer 3 , Susanna Zierler 1 , Vladimir Chubanov 1 , Bernhard Liebl 4 , Thomas Gudermann 1 , Susanne Muehlich 1, 2
Affiliation
|
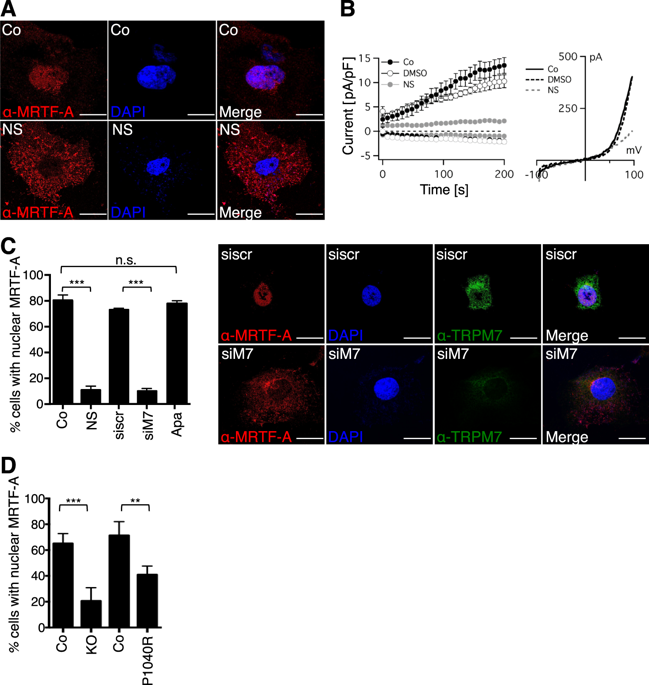
Myocardin-related transcription factors A and B (MRTFs) are coactivators of Serum Response Factor (SRF) that mediates the expression of genes involved in cell proliferation, migration and differentiation. There is mounting evidence that MRTFs and SRF represent promising targets for hepatocellular carcinoma (HCC) growth. Since MRTF-A nuclear localization is a prerequisite for its transcriptional activity and oncogenic properties, we searched for pharmacologically active compounds able to redistribute MRTF-A to the cytoplasm. We identified NS8593, a negative gating modulator of the transient receptor potential cation channel TRPM7, as a novel inhibitor of MRTF-A nuclear localization and transcriptional activity. Using a pharmacological approach and targeted genome editing, we investigated the functional contribution of TRPM7, a unique ion channel containing a serine-threonine kinase domain, to MRTF transcriptional and tumorigenic activity. We found that TRPM7 function regulates RhoA activity and subsequently actin polymerization, MRTF-A-Filamin A complex formation and MRTF-A/SRF target gene expression. Mechanistically, TRPM7 signaling relies on TRPM7 channel-mediated Mg2+ influx and phosphorylation of RhoA by TRPM7 kinase. Pharmacological blockade of TRPM7 results in oncogene-induced senescence of hepatocellular carcinoma (HCC) cells in vitro and in vivo in HCC xenografts. Hence, inhibition of the TRPM7/MRTF axis emerges as a promising strategy to curb HCC growth.
中文翻译:

TRPM7的抑制阻断了MRTF / SRF依赖的转录和致瘤活性。
心肌相关转录因子A和B(MRTF)是血清反应因子(SRF)的共激活因子,它介导参与细胞增殖,迁移和分化的基因的表达。越来越多的证据表明,MRTF和SRF代表了肝细胞癌(HCC)生长的有希望的靶标。由于MRTF-A的核定位是其转录活性和致癌特性的先决条件,因此我们寻找了能够将MRTF-A重新分配到细胞质的药理活性化合物。我们确定NS8593,瞬态受体电位阳离子通道TRPM7的负门控调节剂,作为MRTF-A核定位和转录活性的新型抑制剂。使用药理学方法和针对性的基因组编辑,我们研究了TRPM7的功能性贡献,包含丝氨酸-苏氨酸激酶结构域的独特离子通道,具有MRTF转录和致瘤活性。我们发现TRPM7功能调节RhoA活性和随后的肌动蛋白聚合,MRTF-A-Filamin A复合物的形成和MRTF-A / SRF目标基因的表达。从机制上讲,TRPM7信号依赖于TRPM7通道介导的Mg2 +内流以及TRPM7激酶使RhoA磷酸化。TRPM7的药理学阻断作用导致癌基因诱导的肝癌异种移植体内和体外肝细胞癌(HCC)细胞衰老。因此,抑制TRPM7 / MRTF轴已成为抑制肝癌生长的一种有前途的策略。MRTF-A-Filamin A复合物的形成和MRTF-A / SRF靶基因的表达。从机理上讲,TRPM7信号依赖于TRPM7通道介导的Mg2 +内流以及TRPM7激酶使RhoA磷酸化。TRPM7的药理学阻断作用导致癌基因诱导的肝癌异种移植体内和体外肝细胞癌(HCC)细胞衰老。因此,抑制TRPM7 / MRTF轴已成为抑制肝癌生长的一种有前途的策略。MRTF-A-Filamin A复合物的形成和MRTF-A / SRF靶基因的表达。从机制上讲,TRPM7信号依赖于TRPM7通道介导的Mg2 +内流以及TRPM7激酶使RhoA磷酸化。TRPM7的药理学阻断作用导致癌基因诱导的肝癌异种移植体内和体外肝细胞癌(HCC)细胞衰老。因此,抑制TRPM7 / MRTF轴已成为抑制肝癌生长的一种有前途的策略。
更新日期:2019-12-17
中文翻译:

TRPM7的抑制阻断了MRTF / SRF依赖的转录和致瘤活性。
心肌相关转录因子A和B(MRTF)是血清反应因子(SRF)的共激活因子,它介导参与细胞增殖,迁移和分化的基因的表达。越来越多的证据表明,MRTF和SRF代表了肝细胞癌(HCC)生长的有希望的靶标。由于MRTF-A的核定位是其转录活性和致癌特性的先决条件,因此我们寻找了能够将MRTF-A重新分配到细胞质的药理活性化合物。我们确定NS8593,瞬态受体电位阳离子通道TRPM7的负门控调节剂,作为MRTF-A核定位和转录活性的新型抑制剂。使用药理学方法和针对性的基因组编辑,我们研究了TRPM7的功能性贡献,包含丝氨酸-苏氨酸激酶结构域的独特离子通道,具有MRTF转录和致瘤活性。我们发现TRPM7功能调节RhoA活性和随后的肌动蛋白聚合,MRTF-A-Filamin A复合物的形成和MRTF-A / SRF目标基因的表达。从机制上讲,TRPM7信号依赖于TRPM7通道介导的Mg2 +内流以及TRPM7激酶使RhoA磷酸化。TRPM7的药理学阻断作用导致癌基因诱导的肝癌异种移植体内和体外肝细胞癌(HCC)细胞衰老。因此,抑制TRPM7 / MRTF轴已成为抑制肝癌生长的一种有前途的策略。MRTF-A-Filamin A复合物的形成和MRTF-A / SRF靶基因的表达。从机理上讲,TRPM7信号依赖于TRPM7通道介导的Mg2 +内流以及TRPM7激酶使RhoA磷酸化。TRPM7的药理学阻断作用导致癌基因诱导的肝癌异种移植体内和体外肝细胞癌(HCC)细胞衰老。因此,抑制TRPM7 / MRTF轴已成为抑制肝癌生长的一种有前途的策略。MRTF-A-Filamin A复合物的形成和MRTF-A / SRF靶基因的表达。从机制上讲,TRPM7信号依赖于TRPM7通道介导的Mg2 +内流以及TRPM7激酶使RhoA磷酸化。TRPM7的药理学阻断作用导致癌基因诱导的肝癌异种移植体内和体外肝细胞癌(HCC)细胞衰老。因此,抑制TRPM7 / MRTF轴已成为抑制肝癌生长的一种有前途的策略。









































 京公网安备 11010802027423号
京公网安备 11010802027423号