Nature Catalysis ( IF 42.8 ) Pub Date : 2019-12-16 , DOI: 10.1038/s41929-019-0394-4 Sandra Alonso , Gerard Santiago , Isabel Cea-Rama , Laura Fernandez-Lopez , Cristina Coscolín , Jan Modregger , Anna K. Ressmann , Mónica Martínez-Martínez , Helena Marrero , Rafael Bargiela , Marcos Pita , Jose L. Gonzalez-Alfonso , Manon L. Briand , David Rojo , Coral Barbas , Francisco J. Plou , Peter N. Golyshin , Patrick Shahgaldian , Julia Sanz-Aparicio , Víctor Guallar , Manuel Ferrer
|
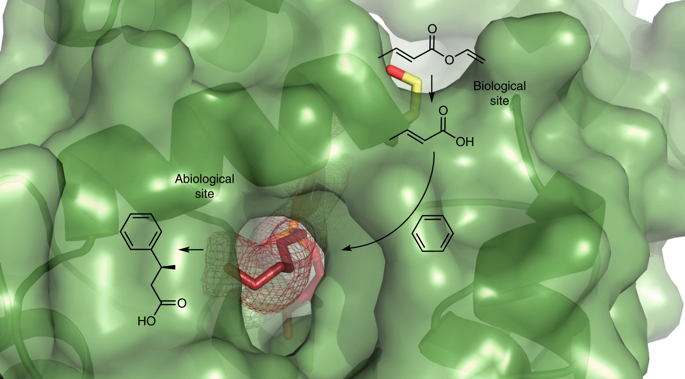
Enzyme engineering has allowed not only the de novo creation of active sites catalysing known biological reactions with rates close to diffusion limits, but also the generation of abiological sites performing new-to-nature reactions. However, the catalytic advantages of engineering multiple active sites into a single protein scaffold are yet to be established. Here, we report on proteins with two active sites of biological and/or abiological origin, for improved natural and non-natural catalysis. The approach increased the catalytic properties, such as enzyme efficiency, substrate scope, stereoselectivity and optimal temperature window, of an esterase containing two biological sites. Then, one of the active sites was metamorphosed into a metal-complex chemocatalytic site for oxidation and Friedel–Crafts alkylation reactions, facilitating synergistic chemo- and biocatalysis in a single protein. The transformations of 1-naphthyl acetate into 1,4-naphthoquinone (conversion approx. 100%) and vinyl crotonate and benzene into 3-phenylbutyric acid (≥83%; e.e. >99.9%) were achieved in one pot with this artificial multifunctional metalloenzyme.
中文翻译:

具有两个活性位点的基因工程蛋白,可增强生物催化作用以及化学和生物催化的协同作用
酶工程不仅允许从头创建活性部位以催化接近扩散极限的已知生物反应,而且还允许生成进行新自然反应的非生物部位。然而,将多个活性位点工程化为单个蛋白质支架的催化优势尚未确立。在这里,我们报告具有两个生物学和/或非生物学来源的活性位点的蛋白质,以改善天然和非天然催化作用。该方法提高了含有两个生物学位点的酯酶的催化性能,例如酶效率,底物范围,立体选择性和最佳温度范围。然后,其中一个活性位点转变为金属复杂的化学催化位点,以进行氧化和Friedel-Crafts烷基化反应,促进单一蛋白质的协同化学和生物催化作用。用这种人工多功能金属酶在一个锅中完成了乙酸1-萘酯向1,4-萘醌的转化(转化率约100%),巴豆酸乙烯酯和苯向3-苯基丁酸的转化率(≥83%; ee> 99.9%)。 。











































 京公网安备 11010802027423号
京公网安备 11010802027423号