Tetrahedron ( IF 2.1 ) Pub Date : 2019-12-13 , DOI: 10.1016/j.tet.2019.130874 Tiago Lima da Silva , Raoni Scheibler Rambo , Caroline Gross Jacoby , Paulo Henrique Schneider
|
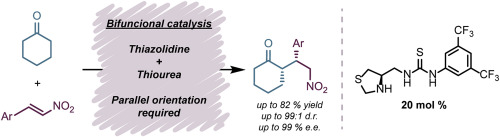
In this work, we report the synthesis and characterization of three new thiazolidine- and thiourea-based chiral organocatalysts. These compounds were successfully applied in asymmetric Michael addition reactions between different ketones and nitrostyrenes leading to products in up to 85% yield, >96:4 r.d. and 97% e.e. Computational studies were used to better visualize the proposed transition state and explain the observed stereoselectivities. One of the new catalysts was also successfully applied in an aldol addition between cyclohexanone an p-nitrobenzaldehyde leading to product in 80% yield, >96:4 d.r. and 80% e.e.
中文翻译:

手性噻唑烷-硫脲催化剂促进不对称迈克尔反应
在这项工作中,我们报告了三种新型基于噻唑烷和硫脲的手性有机催化剂的合成和表征。这些化合物已成功应用于不同的酮与硝基苯乙烯之间的不对称迈克尔加成反应,从而导致产率高达85%,> 96:4 rd和97%ee的计算研究用于更好地可视化拟议的过渡态并解释观察到的立体选择性。一种新的催化剂也成功地用于环己酮和对硝基苯甲醛之间的羟醛加成中,从而以80%的收率,> 96:4 dr和80%ee的产率生产出产品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号