当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical formation of PtRu bimetallic nanoparticles for highly efficient and pH-universal hydrogen evolution reaction†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2019-12-13 , DOI: 10.1039/c9ta12300h Lu Li 1, 2, 3, 4, 5 , Gengwei Zhang 1, 2, 3, 4, 5 , Bin Wang 1, 2, 3, 4, 5 , Tao Yang 1, 2, 3, 4, 5 , Shengchun Yang 1, 2, 3, 4, 5
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2019-12-13 , DOI: 10.1039/c9ta12300h Lu Li 1, 2, 3, 4, 5 , Gengwei Zhang 1, 2, 3, 4, 5 , Bin Wang 1, 2, 3, 4, 5 , Tao Yang 1, 2, 3, 4, 5 , Shengchun Yang 1, 2, 3, 4, 5
Affiliation
|
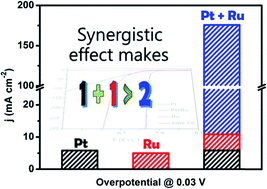
The production of hydrogen by electrolyzing water, which stores the excess temporal fluctuations of solar and wind power in the form of chemical energy, is usually energy intensive and expensive. Therefore, developing highly efficient and low-cost electrocatalysts via a simple synthesis strategy for the hydrogen evolution reaction (HER) has become a remarkable challenge in energy and environmental science. Herein, we report a new simple electrochemical deposition (ECD) method for the synthesis of a highly efficient carbon-cloth-supported PtRu HER catalyst (PtRu/CC1500) in a wide pH range, in which Pt in an ultralow loading amount (1.6 μg cmCC−2) is electrodeposited on Ru nanoparticles (NPs). This catalyst presents remarkable performance, with low overpotentials of only 8, 25 and 19 mV at 10 mA cm−2 (η10) in 0.5 M H2SO4, 1 M phosphate buffer solution (PBS) and 1.0 M KOH, respectively, as well as long-term stability (10 000 cycles). Also, even at a high current density of 300 mA cm−2, it still displays small overpotentials of 37 and 95 mV in 0.5 M H2SO4 and 1.0 M KOH solution, respectively. In addition, the PtRu catalyst achieves an excellent mass current density of 109.9 mA μg−1 (in 0.5 M H2SO4 at −30 mV) and 198.7 mA μg−1 (in 1.0 M KOH at −100 mV), which are 30 and 59 times higher than those of a commercial Pt/C catalyst, respectively. Density functional theory calculations suggest that PtRu nanoparticles (NPs) not only significantly improve hydrogen desorption (ΔGH* very close to zero) but also promote H2O dissociation, accounting for their enhanced catalytic activity. Due to the simple preparation strategy, excellent HER performance and long-term durability of the PtRu catalyst, it has great potential for commercial applications.
中文翻译:

PtRu双金属纳米粒子的电化学形成,可实现高效的pH值通用的析氢反应†
通过电解水生产氢,以化学能的形式存储太阳能和风能的过度时间波动,通常会消耗大量能源,而且价格昂贵。因此,通过简单的氢析出反应(HER)合成策略开发高效,低成本的电催化剂已成为能源与环境科学领域的重大挑战。本文中,我们报道了一种新的简单电化学沉积(ECD)方法,用于在宽pH范围内合成高效碳布负载的PtRu HER催化剂(PtRu / CC 1500),其中Pt的负载量极低(1.6微克cm CC -2)电沉积在Ru纳米颗粒(NPs)上。该催化剂呈现显着的性能,只有8,25和19毫伏低的超电势在10mA厘米-2(η 10)在0.5 MH 2 SO 4,分别的1M磷酸盐缓冲液(PBS)和1.0M KOH,作为以及长期稳定性(10000次循环)。此外,即使在300 mA cm -2的高电流密度下,它在0.5 MH 2 SO 4和1.0 M KOH溶液中仍分别显示出37和95 mV的小过电位。此外,PtRu催化剂具有出色的质量电流密度,为109.9 mAμg -1(在0.5 MH 2 SO 4中)在-30 mV时)和198.7 mAμg -1(在100 mV时在1.0 M KOH中),分别比市售Pt / C催化剂高30倍和59倍。密度泛函理论计算表明,PtRu纳米颗粒(NPs)不仅显着改善了氢的脱附(ΔG H *非常接近于零),而且还促进了H 2 O的离解,这是因为它们具有增强的催化活性。由于简单的制备策略,优异的HER性能和PtRu催化剂的长期耐久性,它具有巨大的商业应用潜力。
更新日期:2020-01-08
中文翻译:

PtRu双金属纳米粒子的电化学形成,可实现高效的pH值通用的析氢反应†
通过电解水生产氢,以化学能的形式存储太阳能和风能的过度时间波动,通常会消耗大量能源,而且价格昂贵。因此,通过简单的氢析出反应(HER)合成策略开发高效,低成本的电催化剂已成为能源与环境科学领域的重大挑战。本文中,我们报道了一种新的简单电化学沉积(ECD)方法,用于在宽pH范围内合成高效碳布负载的PtRu HER催化剂(PtRu / CC 1500),其中Pt的负载量极低(1.6微克cm CC -2)电沉积在Ru纳米颗粒(NPs)上。该催化剂呈现显着的性能,只有8,25和19毫伏低的超电势在10mA厘米-2(η 10)在0.5 MH 2 SO 4,分别的1M磷酸盐缓冲液(PBS)和1.0M KOH,作为以及长期稳定性(10000次循环)。此外,即使在300 mA cm -2的高电流密度下,它在0.5 MH 2 SO 4和1.0 M KOH溶液中仍分别显示出37和95 mV的小过电位。此外,PtRu催化剂具有出色的质量电流密度,为109.9 mAμg -1(在0.5 MH 2 SO 4中)在-30 mV时)和198.7 mAμg -1(在100 mV时在1.0 M KOH中),分别比市售Pt / C催化剂高30倍和59倍。密度泛函理论计算表明,PtRu纳米颗粒(NPs)不仅显着改善了氢的脱附(ΔG H *非常接近于零),而且还促进了H 2 O的离解,这是因为它们具有增强的催化活性。由于简单的制备策略,优异的HER性能和PtRu催化剂的长期耐久性,它具有巨大的商业应用潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号