当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multi-arm Avidin nano-construct for intra-cartilage delivery of small molecule drugs.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-13 , DOI: 10.1016/j.jconrel.2019.12.020 Tengfei He 1 , Chenzhen Zhang 1 , Armin Vedadghavami 1 , Shikhar Mehta 1 , Heather A Clark 2 , Ryan M Porter 3 , Ambika G Bajpayee 4
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-13 , DOI: 10.1016/j.jconrel.2019.12.020 Tengfei He 1 , Chenzhen Zhang 1 , Armin Vedadghavami 1 , Shikhar Mehta 1 , Heather A Clark 2 , Ryan M Porter 3 , Ambika G Bajpayee 4
Affiliation
|
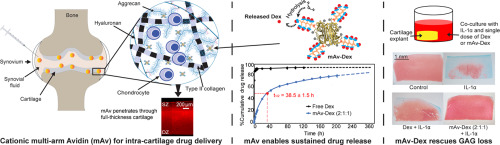
Targeted drug delivery to joint tissues like cartilage remains a challenge that has prevented clinical translation of promising osteoarthritis (OA) drugs. Local intra-articular (IA) injections of drugs suffer from rapid clearance from the joint space and slow diffusive transport through the dense, avascular cartilage matrix comprised of negatively charged glycosaminoglycans (GAGs). Here we apply drug carriers that leverage electrostatic interactions with the tissue's high negative fixed charge density (FCD) for delivering small molecule drugs to cartilage cell and matrix sites. We demonstrate that a multi-arm cationic nano-construct of Avidin (mAv) with 28 sites for covalent drug conjugation can rapidly penetrate through the full thickness of cartilage in high concentration and have long intra-cartilage residence time in both healthy and arthritic cartilage via weak-reversible binding with negatively charged aggrecans. mAv's intra-cartilage mean uptake was found to be 112× and 33× the equilibration bath concentration in healthy and arthritic (50% GAG depleted) cartilage, respectively. mAv was conjugated with Dexamethasone (mAv-Dex), a broad-spectrum glucocorticoid, using a combination of hydrolysable ester linkers derived from succinic anhydride (SA), 3,3-dimethylglutaric anhydride (GA) and phthalic anhydride (PA) in 2:1:1 M ratio that enabled 50% drug release within 38.5 h followed by sustained release in therapeutic doses over 2 weeks. A single 10 μM low dose of controlled release mAv-Dex (2:1:1) effectively suppressed IL-1α-induced GAG loss, cell death and inflammatory response significantly better than unmodified Dex over 2 weeks in cartilage explant culture models of OA. With this multi-arm design, <1 μM Avidin was needed - a concentration which has been shown to be safe, preventing further GAG loss and cytotoxicity. A charge-based cartilage homing drug delivery platform like this can elicit disease modifying effects as well as facilitate long-term symptomatic pain and inflammation relief by enhancing tissue specificity and prolonging intra-cartilage residence time of OA drugs. This nano-construct thus has high translational potential for enabling intra-cartilage delivery of a broad array of small molecule OA drugs and their combinations to chondrocytes, enabling OA treatment with a single injection of low drug doses and eliminating toxicity issues associated with multiple high dose injections.
中文翻译:

多臂抗生物素蛋白纳米结构用于小分子药物的软骨内递送。
靶向药物向软骨等关节组织的靶向递送仍然是一项挑战,已经阻止了有希望的骨关节炎(OA)药物的临床翻译。局部关节内(IA)注射药物会从关节间隙迅速清除,并缓慢扩散通过由带负电荷的糖胺聚糖(GAG)构成的密集的无血管软骨基质。在这里,我们应用药物载体,该药物载体利用静电相互作用与组织的高负固定电荷密度(FCD)将小分子药物递送至软骨细胞和基质部位。我们证明,具有28个共价药物共轭位点的抗生物素蛋白(mAv)的多臂阳离子纳米结构可快速穿透高浓度的整个软骨厚度,并且在健康和关节炎软骨中均具有较长的软骨内滞留时间。带负电荷的聚集蛋白聚糖的弱可逆结合。在健康和关节炎(耗竭GAG的50%)软骨中,mAv的平均软骨内摄取量分别为平衡浴浓度的112倍和33倍。使用衍生自琥珀酸酐(SA),3,3-二甲基戊二酸酐(GA)和邻苯二甲酸酐(PA)的可水解酯连接基的组合,将mAv与广谱糖皮质激素地塞米松(mAv-Dex)偶联: 1:1 M的比例使38%的药物释放50%。5小时,然后以治疗剂量持续释放超过2周。在OA软骨外植体培养模型中,单剂量10μM低剂量的控释mAv-Dex(2:1:1)在2周内能有效抑制IL-1α诱导的GAG丢失,细胞死亡和炎性反应,优于未修饰的Dex。通过这种多臂设计,需要小于1μM的抗生物素蛋白-该浓度已被证明是安全的,可防止进一步的GAG丢失和细胞毒性。这样的基于电荷的软骨归巢药物递送平台可以通过增强组织特异性和延长OA药物在软骨内的停留时间来引起疾病改变效果,并促进长期症状性疼痛和炎症缓解。
更新日期:2019-12-13
中文翻译:

多臂抗生物素蛋白纳米结构用于小分子药物的软骨内递送。
靶向药物向软骨等关节组织的靶向递送仍然是一项挑战,已经阻止了有希望的骨关节炎(OA)药物的临床翻译。局部关节内(IA)注射药物会从关节间隙迅速清除,并缓慢扩散通过由带负电荷的糖胺聚糖(GAG)构成的密集的无血管软骨基质。在这里,我们应用药物载体,该药物载体利用静电相互作用与组织的高负固定电荷密度(FCD)将小分子药物递送至软骨细胞和基质部位。我们证明,具有28个共价药物共轭位点的抗生物素蛋白(mAv)的多臂阳离子纳米结构可快速穿透高浓度的整个软骨厚度,并且在健康和关节炎软骨中均具有较长的软骨内滞留时间。带负电荷的聚集蛋白聚糖的弱可逆结合。在健康和关节炎(耗竭GAG的50%)软骨中,mAv的平均软骨内摄取量分别为平衡浴浓度的112倍和33倍。使用衍生自琥珀酸酐(SA),3,3-二甲基戊二酸酐(GA)和邻苯二甲酸酐(PA)的可水解酯连接基的组合,将mAv与广谱糖皮质激素地塞米松(mAv-Dex)偶联: 1:1 M的比例使38%的药物释放50%。5小时,然后以治疗剂量持续释放超过2周。在OA软骨外植体培养模型中,单剂量10μM低剂量的控释mAv-Dex(2:1:1)在2周内能有效抑制IL-1α诱导的GAG丢失,细胞死亡和炎性反应,优于未修饰的Dex。通过这种多臂设计,需要小于1μM的抗生物素蛋白-该浓度已被证明是安全的,可防止进一步的GAG丢失和细胞毒性。这样的基于电荷的软骨归巢药物递送平台可以通过增强组织特异性和延长OA药物在软骨内的停留时间来引起疾病改变效果,并促进长期症状性疼痛和炎症缓解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号