当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spontaneous Stereoselective Oxidation of Crystalline Avermectin B1a to Its C-8a-(S)-Hydroperoxide.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2019-12-13 , DOI: 10.1021/acs.jnatprod.9b00524 Jan A Gliński 1 , John Proudfoot 2 , Izabela Madura 3 , Huaping Zhang 1 , Michał Gleńsk 1, 4 , Victor Day 5 , Marta K Dudek 6
Journal of Natural Products ( IF 3.3 ) Pub Date : 2019-12-13 , DOI: 10.1021/acs.jnatprod.9b00524 Jan A Gliński 1 , John Proudfoot 2 , Izabela Madura 3 , Huaping Zhang 1 , Michał Gleńsk 1, 4 , Victor Day 5 , Marta K Dudek 6
Affiliation
|
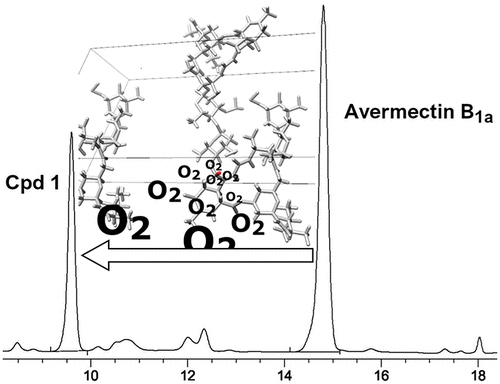
Prolonged storage of technical abamectin as well as avermectin B1a samples yielded a previously unknown derivative, designated here as compound 1. Detailed NMR analysis and X-ray crystallography allowed us to determine the structure of this compound and revealed the presence of a hydroperoxide group (-OOH) attached stereoselectively with configuration S to the C-8a carbon. This surprising result involves the formation of the peroxide bond in solid crystalline avermectin B1a upon exposure to air with no involvement of light or recognized catalytic factors and is consistent with a topotactic mechanism for the oxidation reaction. Compound 1 is stable in the absence of reducing agents and has potential as a starting point in structural modification of the tetrahydrofuran ring of avermectin B1a. It could also serve as a marker in assessing the quality of stored technical abamectin.
中文翻译:

结晶阿维菌素B1a的自发立体选择性氧化为其C-8a-(S)-羟基过氧化物。
长期储存阿维菌素和阿维菌素B1a技术样品会产生以前未知的衍生物,在此称为化合物1。详细的NMR分析和X射线晶体学分析使我们能够确定该化合物的结构,并揭示了氢过氧化物基团(- OOH)以构型S立体选择性地连接到C-8a碳上。该令人惊讶的结果涉及在暴露于空气中而没有光或公认的催化因素的情况下在固体结晶阿维菌素B1a中过氧化物键的形成,并且与氧化反应的全能机理一致。在没有还原剂的情况下,化合物1是稳定的,并且具有作为阿维菌素B1a的四氢呋喃环结构修饰的起点的潜力。
更新日期:2019-12-13
中文翻译:

结晶阿维菌素B1a的自发立体选择性氧化为其C-8a-(S)-羟基过氧化物。
长期储存阿维菌素和阿维菌素B1a技术样品会产生以前未知的衍生物,在此称为化合物1。详细的NMR分析和X射线晶体学分析使我们能够确定该化合物的结构,并揭示了氢过氧化物基团(- OOH)以构型S立体选择性地连接到C-8a碳上。该令人惊讶的结果涉及在暴露于空气中而没有光或公认的催化因素的情况下在固体结晶阿维菌素B1a中过氧化物键的形成,并且与氧化反应的全能机理一致。在没有还原剂的情况下,化合物1是稳定的,并且具有作为阿维菌素B1a的四氢呋喃环结构修饰的起点的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号