当前位置:
X-MOL 学术
›
Energy Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Sodium Polysulfide Battery with Liquid/Solid Electrolyte: Improving Sulfur Utilization Using P2S5 as Additive and Tetramethylurea as Catholyte Solvent
Energy Technology ( IF 3.6 ) Pub Date : 2020-01-09 , DOI: 10.1002/ente.201901200 Lukas Medenbach 1 , Pascal Hartmann 2, 3 , Juergen Janek 3 , Timo Stettner 1 , Andrea Balducci 1 , Cornelius Dirksen 4 , Matthias Schulz 4 , Michael Stelter 4 , Philipp Adelhelm 1, 5, 6
Energy Technology ( IF 3.6 ) Pub Date : 2020-01-09 , DOI: 10.1002/ente.201901200 Lukas Medenbach 1 , Pascal Hartmann 2, 3 , Juergen Janek 3 , Timo Stettner 1 , Andrea Balducci 1 , Cornelius Dirksen 4 , Matthias Schulz 4 , Michael Stelter 4 , Philipp Adelhelm 1, 5, 6
Affiliation
|
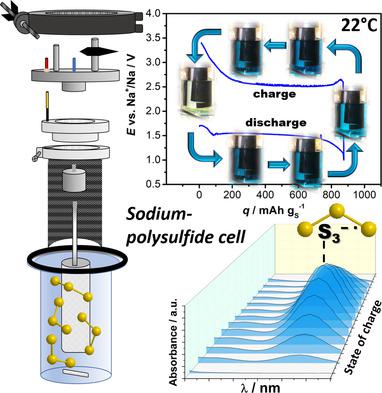
Herein, the proof of concept of a sodium polysulfide battery consisting of two electrode chambers being separated by a solid electrolyte is described. The concept is suited for dissolved polysulfide cathodes and has the advantage that both half reactions can be optimized separately. The formation of solid sulfide discharge products is identified as the major limiting factor for cell cycling. This issue can be alleviated by adding solid P2S5. Further improvement can be achieved by replacing diglyme (2G) as the cathode compartment solvent with tetramethylurea (TMU). Using TMU, the cell cycles with Coulombic efficiencies >99% and capacities of 800 mAh g−1 are maintained for at least 30 cycles. Viscosity, density, conductivity, and the electrochemical stability window values of the 2G‐ and TMU‐based electrolytes are compared. The latter shows higher viscosity (2.806 vs 1.603 mPa s), higher density (1.016 vs 0.996 g cc−1), and higher conductivity (4.27 vs 1.45 mS cm−1). The oxidative stability limit of the TMU electrolyte is 3.2 V versus Na+/Na, which is sufficient for polysulfide redox reactions. Vis spectroscopy is used to follow the electrode reaction. In case of TMU, the reaction is based on the redox activity of S3−• radicals (blue coloration of the catholyte solution).
中文翻译:

具有液体/固体电解质的多硫化钠电池:使用P2S5作为添加剂和四甲基脲作为阴极溶剂来提高硫的利用率
在此,说明由固体电解质隔开的由两个电极室构成的聚硫化钠电池的概念证明。该概念适用于溶解的多硫化物阴极,并具有两个半反应可以分别优化的优点。固体硫化物放电产物的形成被认为是细胞循环的主要限制因素。通过添加固体P 2 S 5可以缓解该问题。通过用四甲基脲(TMU)代替二甘醇二甲醚(2G)作为阴极室溶剂,可以实现进一步的改进。使用TMU,电池循环时库仑效率> 99%,容量为800 mAh g -1至少要维持30个循环。比较了基于2G和TMU的电解质的粘度,密度,电导率和电化学稳定性窗口值。后者显示出更高的粘度(2.806对1.603 mPa s),更高的密度(1.016对0.996 g cc -1)和更高的电导率(4.27对1.45 mS cm -1)。TMU电解质的氧化稳定性极限相对于Na + / Na为3.2 V ,足以进行多硫化物氧化还原反应。可见光谱用于跟踪电极反应。在TMU的情况下,该反应基于S 3- •自由基的氧化还原活性(阴极电解液的蓝色)。
更新日期:2020-01-09
中文翻译:

具有液体/固体电解质的多硫化钠电池:使用P2S5作为添加剂和四甲基脲作为阴极溶剂来提高硫的利用率
在此,说明由固体电解质隔开的由两个电极室构成的聚硫化钠电池的概念证明。该概念适用于溶解的多硫化物阴极,并具有两个半反应可以分别优化的优点。固体硫化物放电产物的形成被认为是细胞循环的主要限制因素。通过添加固体P 2 S 5可以缓解该问题。通过用四甲基脲(TMU)代替二甘醇二甲醚(2G)作为阴极室溶剂,可以实现进一步的改进。使用TMU,电池循环时库仑效率> 99%,容量为800 mAh g -1至少要维持30个循环。比较了基于2G和TMU的电解质的粘度,密度,电导率和电化学稳定性窗口值。后者显示出更高的粘度(2.806对1.603 mPa s),更高的密度(1.016对0.996 g cc -1)和更高的电导率(4.27对1.45 mS cm -1)。TMU电解质的氧化稳定性极限相对于Na + / Na为3.2 V ,足以进行多硫化物氧化还原反应。可见光谱用于跟踪电极反应。在TMU的情况下,该反应基于S 3- •自由基的氧化还原活性(阴极电解液的蓝色)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号