当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluating Electron-Transfer Reactivity of Complexes of Actinides in +2 and +3 Oxidation States by using EPR Spectroscopy.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-12-12 , DOI: 10.1002/chem.201905581 Samuel A Moehring 1 , William J Evans 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2019-12-12 , DOI: 10.1002/chem.201905581 Samuel A Moehring 1 , William J Evans 1
Affiliation
|
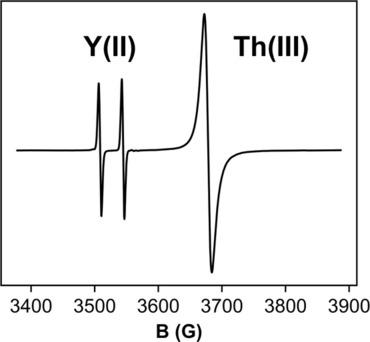
The possibility that the relative reactivity of complexes of actinide metals in the +2 and +3 oxidation states could be investigated by examining reactions between AnIII and AnII species of Th and U with rare-earth metal reagents that provide EPR confirmation of electron transfer reactivity has been explored. Neither Cp''3 ThIII nor Cp''3 UIII will reduce Cp''3 LaIII or Cp'3 YIII (Cp'=C5 H4 SiMe3 , Cp''=C5 H3 (SiMe3 )2 ). However, both [K(2.2.2-cryptand)][Cp''3 ThII ] and [K(2.2.2-cryptand)][Cp''3 UII ] reduce Cp''3 LaIII and Cp'3 YIII to form [Cp''3 LaII ]1- and [Cp'3 YII ]1- , respectively, which were identified by EPR spectroscopy. The reverse reactions also occur which indicates that the reduction potentials are similar. [Cp''3 LaII ]1- reduces Cp'3 YIII and the reverse YII /LaIII combination also occurs. In both cases, the reactions generate EPR spectra indicative of multiple species in the mixtures of LaII and YII , which is consistent with ligand exchange and demonstrates that numerous heteroleptic complexes of these LnII ions exist.
中文翻译:

通过使用EPR光谱评估Act系化合物在+2和+3氧化态下的电子转移反应性。
可以通过检查Th和U的AnIII和AnII物种与稀土金属试剂之间的反应来研究of系金属在+2和+3氧化态下的相对反应性,该反应提供了EPR确认电子转移反应性被探索了。Cp''3 ThIII或Cp''3 UIII都不会还原Cp''3 LaIII或Cp'3 YIII(Cp'= C5 H4 SiMe3,Cp''= C5 H3(SiMe3)2)。但是,[K(2.2.2-cryptand)] [Cp''3 ThII]和[K(2.2.2-cryptand)] [Cp''3 UII]都将Cp''3 LaIII和Cp'3 YIII还原为通过EPR光谱鉴定的形式分别为[Cp'3 LaII] 1-和[Cp'3 YII] 1-。还发生逆反应,这表明还原电位是相似的。[Cp''3 LaII] 1-降低Cp' 3 YIII和反向的YII / LaIII组合也会出现。在这两种情况下,反应均产生EPR光谱,表明LaII和YII混合物中存在多种物质,这与配体交换相符,并证明了这些LnII离子存在许多杂合络合物。
更新日期:2020-01-23
中文翻译:

通过使用EPR光谱评估Act系化合物在+2和+3氧化态下的电子转移反应性。
可以通过检查Th和U的AnIII和AnII物种与稀土金属试剂之间的反应来研究of系金属在+2和+3氧化态下的相对反应性,该反应提供了EPR确认电子转移反应性被探索了。Cp''3 ThIII或Cp''3 UIII都不会还原Cp''3 LaIII或Cp'3 YIII(Cp'= C5 H4 SiMe3,Cp''= C5 H3(SiMe3)2)。但是,[K(2.2.2-cryptand)] [Cp''3 ThII]和[K(2.2.2-cryptand)] [Cp''3 UII]都将Cp''3 LaIII和Cp'3 YIII还原为通过EPR光谱鉴定的形式分别为[Cp'3 LaII] 1-和[Cp'3 YII] 1-。还发生逆反应,这表明还原电位是相似的。[Cp''3 LaII] 1-降低Cp' 3 YIII和反向的YII / LaIII组合也会出现。在这两种情况下,反应均产生EPR光谱,表明LaII和YII混合物中存在多种物质,这与配体交换相符,并证明了这些LnII离子存在许多杂合络合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号