当前位置:
X-MOL 学术
›
Environ. Sci.: Water Res. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organic structure and solid characteristics determine reactivity of phenolic compounds with synthetic and reclaimed manganese oxides
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2019/12/12 , DOI: 10.1039/c9ew00859d Emma Leverich Trainer 1, 2, 3, 4 , Matthew Ginder-Vogel 1, 2, 3, 4, 5 , Christina K. Remucal 1, 2, 3, 4, 5
Environmental Science: Water Research & Technology ( IF 5 ) Pub Date : 2019/12/12 , DOI: 10.1039/c9ew00859d Emma Leverich Trainer 1, 2, 3, 4 , Matthew Ginder-Vogel 1, 2, 3, 4, 5 , Christina K. Remucal 1, 2, 3, 4, 5
Affiliation
|
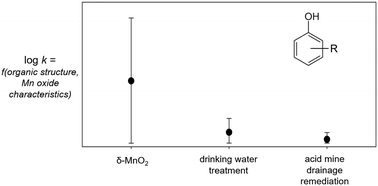
Manganese (Mn) oxides have been proposed for in situ treatment of organic (e.g., phenolic) contaminants, although little is known about the reactivity of reclaimed solids that might be used as alternatives to synthetic oxides. In this study, we investigate the impacts of phenol substituents and manganese oxide properties (e.g., surface area, iron substitution) on the kinetics and mechanism of this reaction. Reclaimed solids from acid mine drainage and drinking water treatment systems contain Mn(IV) and are capable of oxidizing phenolic contaminants, although their reactivity is 1–3 orders of magnitude slower than that of synthetic δ-MnO2. Both electron transfer-limited and sorption-limited mechanisms occur in 29 phenols reacted with the three manganese oxide materials. This finding contrasts with the common assumption that the first one-electron transfer from the phenol to the manganese oxide is rate-limiting. The occurrence of both mechanisms has implications for the rates and products of phenol oxidation. Interestingly, the mechanism for a given phenol changes between solids. We attribute this observed mechanism shift primarily to phenolic substituent effects, with influences from the pHpzc, surface area, and iron substitution of the manganese oxide materials. In addition, we investigate the predictive utility of quantitative structure–activity relationships, as these models have not been tested using complex reactants and non-synthetic manganese oxides. In-depth analysis and external validation measures indicate these common QSAR models are ineffective at predicting the behavior of complex contaminants or reactions with non-synthetic manganese oxides, and therefore have limited application for predicting contaminant oxidation by manganese oxides in environmental and engineered systems.
中文翻译:

有机结构和固体特性决定了酚类化合物与合成和再生锰氧化物的反应性
已经提出了锰(Mn)氧化物用于有机(例如酚醛)污染物的原位处理,尽管对于可以用作合成氧化物的替代物的再生固体的反应性知之甚少。在这项研究中,我们研究了苯酚取代基和氧化锰特性(例如表面积,铁取代)对反应动力学和机理的影响。从酸性矿山排水和饮用水处理系统回收固体含有的Mn(IV)和能够氧化酚污染物的,虽然它们的反应性比合成的较慢的1-3个数量级δ-的MnO 2。与三种锰氧化物材料反应的29种苯酚中均发生电子传递受限和吸附受限的机理。该发现与通常的假设相反,该假设是从苯酚到氧化锰的第一次单电子转移是限速的。两种机理的发生都对苯酚氧化的速率和产物有影响。有趣的是,给定苯酚的机理在固体之间会发生变化。我们将这种观察到的机理转变主要归因于酚取代基的影响,并受pH pzc的影响,表面积和氧化锰材料的铁替代物。此外,我们还研究了定量结构与活性关系的预测效用,因为这些模型尚未使用复杂的反应物和非合成锰氧化物进行过测试。深入的分析和外部验证措施表明,这些常见的QSAR模型在预测复杂污染物的行为或与非合成锰氧化物的反应方面无效,因此在预测环境和工程系统中锰氧化物的污染物氧化方面应用有限。
更新日期:2020-03-05
中文翻译:

有机结构和固体特性决定了酚类化合物与合成和再生锰氧化物的反应性
已经提出了锰(Mn)氧化物用于有机(例如酚醛)污染物的原位处理,尽管对于可以用作合成氧化物的替代物的再生固体的反应性知之甚少。在这项研究中,我们研究了苯酚取代基和氧化锰特性(例如表面积,铁取代)对反应动力学和机理的影响。从酸性矿山排水和饮用水处理系统回收固体含有的Mn(IV)和能够氧化酚污染物的,虽然它们的反应性比合成的较慢的1-3个数量级δ-的MnO 2。与三种锰氧化物材料反应的29种苯酚中均发生电子传递受限和吸附受限的机理。该发现与通常的假设相反,该假设是从苯酚到氧化锰的第一次单电子转移是限速的。两种机理的发生都对苯酚氧化的速率和产物有影响。有趣的是,给定苯酚的机理在固体之间会发生变化。我们将这种观察到的机理转变主要归因于酚取代基的影响,并受pH pzc的影响,表面积和氧化锰材料的铁替代物。此外,我们还研究了定量结构与活性关系的预测效用,因为这些模型尚未使用复杂的反应物和非合成锰氧化物进行过测试。深入的分析和外部验证措施表明,这些常见的QSAR模型在预测复杂污染物的行为或与非合成锰氧化物的反应方面无效,因此在预测环境和工程系统中锰氧化物的污染物氧化方面应用有限。



























 京公网安备 11010802027423号
京公网安备 11010802027423号