当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Switching of regioselectivity in base-mediated diastereoselective annulation of 2,3-epoxy tosylates and their N-tosylaziridine analogs with 2-mercaptobenzimidazole.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9ob02454a Arup Jyoti Das 1 , Hemi Borgohain , Bipul Sarma , Sajal Kumar Das
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9ob02454a Arup Jyoti Das 1 , Hemi Borgohain , Bipul Sarma , Sajal Kumar Das
Affiliation
|
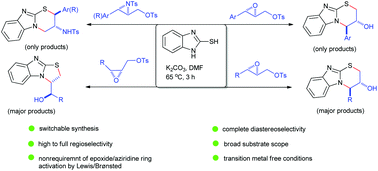
A base-mediated dinucleophilic cyclization of readily accessible 2,3-epoxy tosylates with 2-mercaptobenzimidazole has been developed for the one-pot diastereoselective synthesis of benzimidazole-based tricyclic compounds equipped with two stereogenic centres. With trans-substrates bearing an aryl or alkyl substituent at the C3 position, the reaction involves an initial S-C1 bond-forming intermolecular alkylation followed by an N-C3 bond-forming, endo-selective intramolecular epoxide ring-opening cyclization reaction. A spectacular regioselectivity switching (tandem S-C3 and N-C1 bond formation reactions) was observed with related trans-N-tosylaziridine substrates. Wide substrate scope, complete diastereoselectivity, high to complete regioselectivity and mild transition metal-free conditions render this protocol particularly efficient and practical.
中文翻译:

2,3-环氧甲苯磺酸盐及其N-甲苯磺酰氮丙啶类似物与2-巯基苯并咪唑的碱介导的非对映选择性环空中区域选择性的切换。
已开发出一种由碱介导的易于接近的2,3-环氧甲苯磺酸盐与2-巯基苯并咪唑的二亲核环化反应,用于一锅非对映选择性合成基于苯并咪唑的三环化合物,该化合物具有两个立体异构中心。对于在C3位置带有芳基或烷基取代基的反式底物,该反应涉及初始的形成S-C1键的分子间烷基化,然后进行形成N-C3键的内选择性分子内环氧化物开环环化反应。在相关的反式-N-甲苯磺酰氮丙啶底物上观察到了惊人的区域选择性转换(串联S-C3和N-C1键形成反应)。宽的底物范围,完全的非对映选择性,高至完全的区域选择性以及温和的无过渡金属条件,使得该方案特别有效和实用。
更新日期:2020-01-02
中文翻译:

2,3-环氧甲苯磺酸盐及其N-甲苯磺酰氮丙啶类似物与2-巯基苯并咪唑的碱介导的非对映选择性环空中区域选择性的切换。
已开发出一种由碱介导的易于接近的2,3-环氧甲苯磺酸盐与2-巯基苯并咪唑的二亲核环化反应,用于一锅非对映选择性合成基于苯并咪唑的三环化合物,该化合物具有两个立体异构中心。对于在C3位置带有芳基或烷基取代基的反式底物,该反应涉及初始的形成S-C1键的分子间烷基化,然后进行形成N-C3键的内选择性分子内环氧化物开环环化反应。在相关的反式-N-甲苯磺酰氮丙啶底物上观察到了惊人的区域选择性转换(串联S-C3和N-C1键形成反应)。宽的底物范围,完全的非对映选择性,高至完全的区域选择性以及温和的无过渡金属条件,使得该方案特别有效和实用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号