当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spin-orbit coupling is the key to unraveling intriguing features of the halogen bond involving astatine.
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cp06293a Elisa Rossi 1 , Matteo De Santis 1 , Diego Sorbelli 1 , Loriano Storchi 2 , Leonardo Belpassi 3 , Paola Belanzoni 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-01-08 , DOI: 10.1039/c9cp06293a Elisa Rossi 1 , Matteo De Santis 1 , Diego Sorbelli 1 , Loriano Storchi 2 , Leonardo Belpassi 3 , Paola Belanzoni 4
Affiliation
|
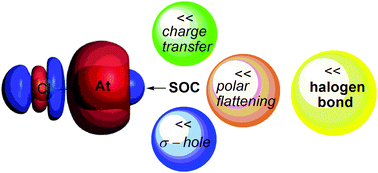
The effect of spin-orbit coupling (SOC) on the halogen bond involving astatine has been investigated using state-of-the-art two- and four-component relativistic calculations. Adducts between Cl-X (X = Cl, Br, I and At) and ammonia have been selected to establish a trend on going down the periodic table. The SOC influence has been explored not only on the geometric and energetic features that can be used to characterize the halogen bond strength but also on the three main contributions to it that are the charge transfer, the "σ-hole" (i.e. the localized region with a net positive electrostatic potential at the halogen site) and the "polar flattening" (which is related to the effective shape of the halogen site). A surprisingly large increase of the Cl-At dipole moment, due to the inclusion of SOC, has been worked out using four-component CCSD(T) reference calculations, indicating that this bond is significantly more ionic than one may predict. Due to the SOC effect, which induces a peculiar charge accumulation on the At side in the Cl-At dimer, a weakening of the astatine-mediated halogen bond occurs arising from the (i) reduced amount of charge transfer, (ii) decrease of the polar flattening and (iii) lowering of the short-range Coulomb potential. The analysis of the electronic structure of the Cl-At moiety allows for a rationalization of the SOC effects on all the considered features of the halogen bond, including an unprecedented unsymmetrical charge back-donation from Cl-At to ammonia.
中文翻译:

自旋轨道耦合是揭示涉及involving的卤素键有趣特征的关键。
自旋轨道耦合(SOC)对涉及a的卤素键的影响已使用最新的两分量和四分量相对论计算进行了研究。选择了Cl-X(X = Cl,Br,I和At)与氨之间的加合物,以确定元素周期表下降的趋势。不仅在可用来表征卤素键强度的几何和高能特征方面探索了SOC影响,而且还研究了电荷的三个主要贡献,即“σ空穴”(即局部区域)对SOC的影响。在卤素位点具有净正静电势)和“极性平坦化”(与卤素位点的有效形状有关)。由于加入了SOC,Cl-At偶极矩出现了惊人的大幅增加,已经使用四组分CCSD(T)参考计算得出了该结果,表明该键的离子化程度大大超过人们的预期。由于SOC效应,会在Cl-At二聚体的At侧引起特殊的电荷积聚,这是由于(i)电荷转移量减少,(ii)电荷转移量减少而引起的-介导的卤素键变弱。极化变平;(iii)降低短程库仑电势。对Cl-At部分电子结构的分析可以合理地考虑到SOC对卤素键所有已考虑特征的影响,包括前所未有的从Cl-At到氨的不对称电荷回供。会在Cl-At二聚体的At侧引起特殊的电荷积聚,这是由于(i)电荷转移量减少,(ii)极性平坦化降低和(iii)降低了a素介导的卤素键)降低短程库仑电势。对Cl-At部分电子结构的分析可以合理地考虑SOC对卤素键所有考虑到的特征的影响,包括前所未有的从Cl-At到氨的不对称电荷反馈。会在Cl-At二聚体的At侧引起特殊的电荷积聚,这是由于(i)电荷转移量减少,(ii)极性平坦化降低和(iii)降低了a素介导的卤素键)降低短程库仑电势。对Cl-At部分电子结构的分析可以合理地考虑SOC对卤素键所有考虑到的特征的影响,包括前所未有的从Cl-At到氨的不对称电荷反馈。
更新日期:2020-01-08
中文翻译:

自旋轨道耦合是揭示涉及involving的卤素键有趣特征的关键。
自旋轨道耦合(SOC)对涉及a的卤素键的影响已使用最新的两分量和四分量相对论计算进行了研究。选择了Cl-X(X = Cl,Br,I和At)与氨之间的加合物,以确定元素周期表下降的趋势。不仅在可用来表征卤素键强度的几何和高能特征方面探索了SOC影响,而且还研究了电荷的三个主要贡献,即“σ空穴”(即局部区域)对SOC的影响。在卤素位点具有净正静电势)和“极性平坦化”(与卤素位点的有效形状有关)。由于加入了SOC,Cl-At偶极矩出现了惊人的大幅增加,已经使用四组分CCSD(T)参考计算得出了该结果,表明该键的离子化程度大大超过人们的预期。由于SOC效应,会在Cl-At二聚体的At侧引起特殊的电荷积聚,这是由于(i)电荷转移量减少,(ii)电荷转移量减少而引起的-介导的卤素键变弱。极化变平;(iii)降低短程库仑电势。对Cl-At部分电子结构的分析可以合理地考虑到SOC对卤素键所有已考虑特征的影响,包括前所未有的从Cl-At到氨的不对称电荷回供。会在Cl-At二聚体的At侧引起特殊的电荷积聚,这是由于(i)电荷转移量减少,(ii)极性平坦化降低和(iii)降低了a素介导的卤素键)降低短程库仑电势。对Cl-At部分电子结构的分析可以合理地考虑SOC对卤素键所有考虑到的特征的影响,包括前所未有的从Cl-At到氨的不对称电荷反馈。会在Cl-At二聚体的At侧引起特殊的电荷积聚,这是由于(i)电荷转移量减少,(ii)极性平坦化降低和(iii)降低了a素介导的卤素键)降低短程库仑电势。对Cl-At部分电子结构的分析可以合理地考虑SOC对卤素键所有考虑到的特征的影响,包括前所未有的从Cl-At到氨的不对称电荷反馈。











































 京公网安备 11010802027423号
京公网安备 11010802027423号