当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stimuli-responsive polymeric prodrug-based nanomedicine delivering nifuroxazide and doxorubicin against primary breast cancer and pulmonary metastasis.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-12 , DOI: 10.1016/j.jconrel.2019.12.017 Lei Luo 1 , Fanshu Xu 1 , Huilan Peng 1 , Yonghuang Luo 1 , Xiaohe Tian 2 , Giuseppe Battaglia 3 , Hu Zhang 4 , Qiyong Gong 5 , Zhongwei Gu 5 , Kui Luo 5
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2019-12-12 , DOI: 10.1016/j.jconrel.2019.12.017 Lei Luo 1 , Fanshu Xu 1 , Huilan Peng 1 , Yonghuang Luo 1 , Xiaohe Tian 2 , Giuseppe Battaglia 3 , Hu Zhang 4 , Qiyong Gong 5 , Zhongwei Gu 5 , Kui Luo 5
Affiliation
|
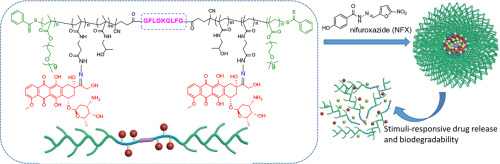
Functionalized drug delivery systems against malignant lung metastasis of breast cancer have been extensively studied, while metastasis remains a challenging issue. We propose a new strategy to achieve eradication of primary breast cancer cells and inhibition of pulmonary metastasis. A cathepsin B/pH dual-sensitive block copolymer with a molecular weight of 92 kDa was synthesized to conjugate with doxorubicin (DOX). The copolymer-DOX was further loaded with nifuroxazide (NFX) to self-assemble co-prodrug-loaded micelles (CLM). CLM displayed a drug release pattern in response to pH/enzyme dual stimuli and was enzymatically biodegradable. CLM was demonstrated to reduce viability and inhibit migration and invasion of 4T1 murine breast cancer cells in vitro. After i.v. injection of CLM, its nanoscale size and stimuli-responsiveness facilitated delivery of drugs to the tumor site in mice. Enhanced anti-tumor efficacy and great anti-metastatic effects were found in both orthotropic and lung metastasis 4T1 breast cancer mice models. Meanwhile, histological immunofluorescence and immunohistochemical analyses revealed a high level of apoptosis, suppressed expression of matrix metalloproteinases and reduction in MDSCs infiltration, and all these contributed to inhibit pulmonary metastasis. CLM may be explored as a potential nanomedicine against breast cancer metastasis.
中文翻译:

刺激响应性的基于聚合物前药的纳米药物,可递送尼呋拉嗪和阿霉素对抗原发性乳腺癌和肺转移。
针对乳腺癌的恶性肺转移的功能化药物递送系统已被广泛研究,而转移仍然是一个具有挑战性的问题。我们提出了一种新的策略,以根除原发性乳腺癌细胞并抑制肺转移。合成了分子量为92 kDa的组织蛋白酶B / pH双敏感嵌段共聚物,使其与阿霉素(DOX)结合。共聚物-DOX进一步负载了呋喃嗪(NFX),以自组装负载前药的胶束(CLM)。CLM响应pH /酶双重刺激而显示出药物释放模式,并且可以酶促生物降解。已证明CLM在体外可降低活力并抑制4T1鼠乳腺癌细胞的迁移和侵袭。静脉注射CLM后,它的纳米级尺寸和刺激反应性促进了药物向小鼠肿瘤部位的递送。在正交各向异性和肺转移4T1乳腺癌小鼠模型中均发现增强的抗肿瘤功效和强大的抗转移作用。同时,组织学免疫荧光和免疫组织化学分析显示出高水平的细胞凋亡,抑制了基质金属蛋白酶的表达和减少了MDSCs的渗透,所有这些都有助于抑制肺转移。CLM可作为一种潜在的针对乳腺癌转移的纳米药物进行研究。组织学免疫荧光和免疫组织化学分析显示高水平的凋亡,抑制基质金属蛋白酶的表达和减少MDSCs渗透,所有这些都有助于抑制肺转移。CLM可作为一种潜在的针对乳腺癌转移的纳米药物进行研究。组织学免疫荧光和免疫组织化学分析显示高水平的凋亡,抑制基质金属蛋白酶的表达和减少MDSCs渗透,所有这些都有助于抑制肺转移。CLM可作为一种潜在的针对乳腺癌转移的纳米药物进行研究。
更新日期:2019-12-13
中文翻译:

刺激响应性的基于聚合物前药的纳米药物,可递送尼呋拉嗪和阿霉素对抗原发性乳腺癌和肺转移。
针对乳腺癌的恶性肺转移的功能化药物递送系统已被广泛研究,而转移仍然是一个具有挑战性的问题。我们提出了一种新的策略,以根除原发性乳腺癌细胞并抑制肺转移。合成了分子量为92 kDa的组织蛋白酶B / pH双敏感嵌段共聚物,使其与阿霉素(DOX)结合。共聚物-DOX进一步负载了呋喃嗪(NFX),以自组装负载前药的胶束(CLM)。CLM响应pH /酶双重刺激而显示出药物释放模式,并且可以酶促生物降解。已证明CLM在体外可降低活力并抑制4T1鼠乳腺癌细胞的迁移和侵袭。静脉注射CLM后,它的纳米级尺寸和刺激反应性促进了药物向小鼠肿瘤部位的递送。在正交各向异性和肺转移4T1乳腺癌小鼠模型中均发现增强的抗肿瘤功效和强大的抗转移作用。同时,组织学免疫荧光和免疫组织化学分析显示出高水平的细胞凋亡,抑制了基质金属蛋白酶的表达和减少了MDSCs的渗透,所有这些都有助于抑制肺转移。CLM可作为一种潜在的针对乳腺癌转移的纳米药物进行研究。组织学免疫荧光和免疫组织化学分析显示高水平的凋亡,抑制基质金属蛋白酶的表达和减少MDSCs渗透,所有这些都有助于抑制肺转移。CLM可作为一种潜在的针对乳腺癌转移的纳米药物进行研究。组织学免疫荧光和免疫组织化学分析显示高水平的凋亡,抑制基质金属蛋白酶的表达和减少MDSCs渗透,所有这些都有助于抑制肺转移。CLM可作为一种潜在的针对乳腺癌转移的纳米药物进行研究。











































 京公网安备 11010802027423号
京公网安备 11010802027423号