Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ParB-type DNA Segregation Proteins Are CTP-Dependent Molecular Switches.
Cell ( IF 45.5 ) Pub Date : 2019-12-12 , DOI: 10.1016/j.cell.2019.11.015 Manuel Osorio-Valeriano 1 , Florian Altegoer 2 , Wieland Steinchen 2 , Svenja Urban 3 , Ying Liu 3 , Gert Bange 2 , Martin Thanbichler 4
Cell ( IF 45.5 ) Pub Date : 2019-12-12 , DOI: 10.1016/j.cell.2019.11.015 Manuel Osorio-Valeriano 1 , Florian Altegoer 2 , Wieland Steinchen 2 , Svenja Urban 3 , Ying Liu 3 , Gert Bange 2 , Martin Thanbichler 4
Affiliation
|
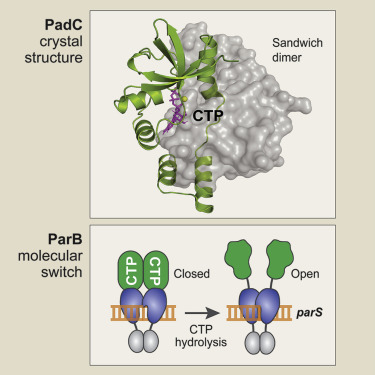
During cell division, newly replicated DNA is actively segregated to the daughter cells. In most bacteria, this process involves the DNA-binding protein ParB, which condenses the centromeric regions of sister DNA molecules into kinetochore-like structures that recruit the DNA partition ATPase ParA and the prokaroytic SMC/condensin complex. Here, we report the crystal structure of a ParB-like protein (PadC) that emerges to tightly bind the ribonucleotide CTP. The CTP-binding pocket of PadC is conserved in ParB and composed of signature motifs known to be essential for ParB function. We find that ParB indeed interacts with CTP and requires nucleotide binding for DNA condensation in vivo. We further show that CTP-binding modulates the affinity of ParB for centromeric parS sites, whereas parS recognition stimulates its CTPase activity. ParB proteins thus emerge as a new class of CTP-dependent molecular switches that act in concert with ATPases and GTPases to control fundamental cellular functions.
中文翻译:

ParB型DNA分离蛋白是CTP依赖的分子开关。
在细胞分裂过程中,新复制的DNA被主动分离到子细胞中。在大多数细菌中,该过程涉及DNA结合蛋白ParB,该蛋白将姐妹DNA分子的着丝粒区域凝结成动粒状结构,从而募集DNA分区ATPase ParA和原核SMC /凝集素复合物。在这里,我们报告一个ParB样蛋白(PadC)的晶体结构出现,它紧紧地结合了核糖核苷酸CTP。PadC的CTP结合袋在ParB中保存,并由已知对ParB功能必不可少的签名图案组成。我们发现,ParB确实与CTP相互作用,并且需要核苷酸结合才能在体内进行DNA缩合。我们进一步表明,CTP结合调节ParB对着丝粒parS站点的亲和力,而parS识别则刺激其CTPase活性。
更新日期:2019-12-13
中文翻译:

ParB型DNA分离蛋白是CTP依赖的分子开关。
在细胞分裂过程中,新复制的DNA被主动分离到子细胞中。在大多数细菌中,该过程涉及DNA结合蛋白ParB,该蛋白将姐妹DNA分子的着丝粒区域凝结成动粒状结构,从而募集DNA分区ATPase ParA和原核SMC /凝集素复合物。在这里,我们报告一个ParB样蛋白(PadC)的晶体结构出现,它紧紧地结合了核糖核苷酸CTP。PadC的CTP结合袋在ParB中保存,并由已知对ParB功能必不可少的签名图案组成。我们发现,ParB确实与CTP相互作用,并且需要核苷酸结合才能在体内进行DNA缩合。我们进一步表明,CTP结合调节ParB对着丝粒parS站点的亲和力,而parS识别则刺激其CTPase活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号