当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of novel 5-substituted-2-aminotetralin analogs: 5-HT1A and 5-HT7 G protein-coupled receptor affinity, 3D-QSAR and molecular modeling.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-12-12 , DOI: 10.1016/j.bmc.2019.115262 Charles K Perry 1 , Austen B Casey 1 , Daniel E Felsing 1 , Rajender Vemula 1 , Mehreen Zaka 1 , Noah B Herrington 2 , Meng Cui 1 , Glen E Kellogg 2 , Clinton E Canal 1 , Raymond G Booth 1
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-12-12 , DOI: 10.1016/j.bmc.2019.115262 Charles K Perry 1 , Austen B Casey 1 , Daniel E Felsing 1 , Rajender Vemula 1 , Mehreen Zaka 1 , Noah B Herrington 2 , Meng Cui 1 , Glen E Kellogg 2 , Clinton E Canal 1 , Raymond G Booth 1
Affiliation
|
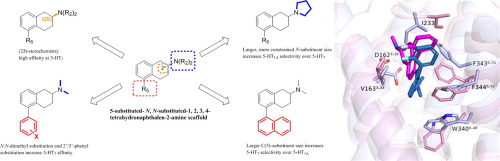
The serotonin 5-HT7 G protein-coupled receptor (GPCR) is a proposed pharmacotherapeutic target for a variety of central and peripheral indications, albeit, there are no approved drugs selective for binding 5-HT7. We previously reported that a lead analog based on the 5-substituted-N,N-disubstituted-1,2,3,4-tetrahydronaphthalen-2-amine (5-substituted-2-aminotetralin, 5-SAT) scaffold binds with high affinity at the 5-HT7 GPCR, and can treat symptoms of autism in mouse models; subsequently, the lead was found to have high affinity at the 5-HT1A GPCR. Herein, we report the synthesis of novel 5-SAT analogs to develop a 3-dimensional quantitative structure-affinity relationship (3D-QSAR) at the human 5-HT7 receptor for comparison with similar studies at the highly homologous 5-HT1A receptor. We report 35 new 5-SAT ligands, some with very high affinity (Ki ≤ 1 nM) and stereoselectivity at 5-HT7 + or 5-HT1A receptors, several with modest selectivity (up to 12-fold) for binding at 5-HT7, and, several ligands with high selectivity (up to 40-fold) at the 5-HT1A receptor. 3D-QSAR results indicate that steric extensions at the C(5)-position improve selectivity for the 5-HT7 over 5-HT1A receptor, while steric and hydrophobic extensions at the chiral C(2)-amino position impart 5-HT1A selectivity. In silico receptor homology modeling studies, supplemented with molecular dynamics simulations and binding free energy calculations, were used to rationalize experimentally-determined receptor selectivity and stereoselective affinity results. The data from these studies indicate that the 5-SAT chemotype, previously shown to be safe and efficacious in rodent paradigms of neurodevelopmental and neuropsychiatric disorders, is amenable to structural modification to optimize affinity at serotonin 5-HT7 vs. 5-HT1A GPCRs, as may be required for successful clinical translation.
中文翻译:

新型5-取代-2-氨基四氢萘类似物的合成:5-HT1A和5-HT7 G蛋白偶联的受体亲和力,3D-QSAR和分子建模。
血清素5-HT7 G蛋白偶联受体(GPCR)是针对各种中枢和外周适应症的拟议药物治疗靶标,尽管尚无批准的选择性结合5-HT7的药物。我们先前曾报道,基于5-取代-N,N-二取代-1,2,3,4-四氢萘-2-胺(5-取代-2-氨基四氢萘,5-SAT)支架的前导类似物与在5-HT7 GPCR上具有亲和力,并且可以治疗小鼠模型中的自闭症症状;随后,发现该铅对5-HT1A GPCR具有高亲和力。在这里,我们报告了新型5-SAT类似物的合成,以在人5-HT7受体上建立三维定量结构亲和关系(3D-QSAR),用于与在高度同源的5-HT1A受体上进行的类似研究进行比较。我们报告了35种新的5-SAT配体,一些对5-HT7 +或5-HT1A受体具有极高的亲和力(Ki≤1 nM)和立体选择性,另一些对与5-HT7结合具有适度的选择性(最高12倍),而一些对配体的选择性高(在5-HT1A受体上最多可扩增40倍)。3D-QSAR结果表明,在C(5)位置的空间延伸提高了对5-HT7的选择性,超过了5-HT1A受体,而在手性C(2)-氨基位置的空间和疏水延伸赋予了5-HT1A选择性。在硅受体同源性建模研究中,辅以分子动力学模拟和结合自由能计算,用于合理化实验确定的受体选择性和立体选择性亲和力结果。这些研究的数据表明,5-SAT化学型
更新日期:2019-12-13
中文翻译:

新型5-取代-2-氨基四氢萘类似物的合成:5-HT1A和5-HT7 G蛋白偶联的受体亲和力,3D-QSAR和分子建模。
血清素5-HT7 G蛋白偶联受体(GPCR)是针对各种中枢和外周适应症的拟议药物治疗靶标,尽管尚无批准的选择性结合5-HT7的药物。我们先前曾报道,基于5-取代-N,N-二取代-1,2,3,4-四氢萘-2-胺(5-取代-2-氨基四氢萘,5-SAT)支架的前导类似物与在5-HT7 GPCR上具有亲和力,并且可以治疗小鼠模型中的自闭症症状;随后,发现该铅对5-HT1A GPCR具有高亲和力。在这里,我们报告了新型5-SAT类似物的合成,以在人5-HT7受体上建立三维定量结构亲和关系(3D-QSAR),用于与在高度同源的5-HT1A受体上进行的类似研究进行比较。我们报告了35种新的5-SAT配体,一些对5-HT7 +或5-HT1A受体具有极高的亲和力(Ki≤1 nM)和立体选择性,另一些对与5-HT7结合具有适度的选择性(最高12倍),而一些对配体的选择性高(在5-HT1A受体上最多可扩增40倍)。3D-QSAR结果表明,在C(5)位置的空间延伸提高了对5-HT7的选择性,超过了5-HT1A受体,而在手性C(2)-氨基位置的空间和疏水延伸赋予了5-HT1A选择性。在硅受体同源性建模研究中,辅以分子动力学模拟和结合自由能计算,用于合理化实验确定的受体选择性和立体选择性亲和力结果。这些研究的数据表明,5-SAT化学型











































 京公网安备 11010802027423号
京公网安备 11010802027423号