Communications Chemistry ( IF 5.9 ) Pub Date : 2019-12-12 , DOI: 10.1038/s42004-019-0242-0 Yi-Yang Zhan , Qi-Chun Jiang , Kentaro Ishii , Takuya Koide , Osamu Kobayashi , Tatsuo Kojima , Satoshi Takahashi , Masanori Tachikawa , Susumu Uchiyama , Shuichi Hiraoka
|
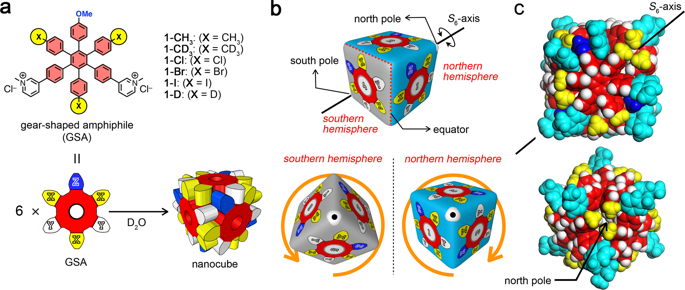
True understanding of dispersion interaction in solution remains elusive because of difficulty in the precise evaluation of its interaction energy. Here, the effect of substituents with different polarizability on dispersion interactions in water is discussed based on the thermodynamic parameters determined by isothermal titration calorimetry for the formation of discrete aggregates from gear-shaped amphiphiles (GSAs). The substituents with higher polarizability enthalpically more stabilize the nanocube, which is due to stronger dispersion interactions and to the hydrophobic effect. The differences in the thermodynamic parameters for the nanocubes from the GSAs with CH3 and CD3 groups are also discussed to lead to the conclusion that the H/D isotope effect on dispersion interactions is negligibly small, which is due to almost perfect entropy-enthalpy compensation between the two isotopomers.
中文翻译:

极化率和同位素对水中分散相互作用的影响
由于很难准确评估溶液中的相互作用能,因此对溶液中分散体相互作用的真正了解仍然难以捉摸。在此,基于等温滴定量热法测定的热力学参数,讨论了不同极化度的取代基对水中分散体相互作用的影响,该热力学参数用于由齿轮形两亲物(GSA)形成离散的聚集体。具有较高极化率的取代基在焓上更稳定纳米立方体,这是由于较强的分散相互作用和疏水作用所致。具有CH 3和CD 3的GSA纳米立方体的热力学参数差异 还讨论了两个基团,得出结论:H / D同位素对分散相互作用的影响可忽略不计,这是由于两个同位异构体之间几乎完美的熵焓补偿所致。



























 京公网安备 11010802027423号
京公网安备 11010802027423号