当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
ATP Controls the Aggregation of Aβ16-22 Peptides.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jpcb.9b10175 Saikat Pal 1 , Sandip Paul 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-12-19 , DOI: 10.1021/acs.jpcb.9b10175 Saikat Pal 1 , Sandip Paul 1
Affiliation
|
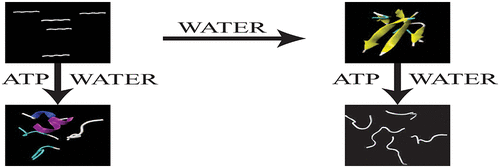
The oligomerization of Aβ16-22 peptide, which is the hydrophobic core region of full-length Aβ1-42, causes Alzheimer's disease (AD). This progressive neurodegenerative disease affects over 44 million people worldwide. However, very few synthesized drug molecules are available to inhibit the aggregation of Aβ. Recently, experimental studies have shown that the biological ATP molecule prevents Aβ fibrillation at the millimolar scale; however, the significance of ATP molecules on Aβ fibrillation and the mechanism behind it remain elusive. We have carried out a total of 7.5 μs extensive all-atom molecular dynamics and 8.82 μs of umbrella sampling in explicit water using AMBER14SB, AMBER99SB-ILDN, and AMBER-FB15 force fields for Aβ16-22 peptide, to investigate the role of ATP on the disruption of Aβ16-22 prefibrils. From various analyses, such as secondary structure analysis, residue-wise contact map, SASA, and interaction energies, we have observed that, in the presence of ATP, the aggregation of Aβ16-22 peptide is very unfavorable. Moreover, the biological molecule ATP interacts with the Aβ16-22 peptide via hydrogen bonding, π-π stacking, and NH-π interactions which, ultimately, prevent the aggregation of Aβ16-22 peptide. Hence, we assume that the deficiency of ATP may cause Alzheimer's disease (AD).
中文翻译:

ATP控制Aβ16-22肽的聚集。
作为全长Aβ1-42的疏水核心区域的Aβ16-22肽的寡聚引起阿尔茨海默氏病(AD)。这种进行性神经退行性疾病影响全世界超过4400万人。但是,很少有合成的药物分子可用于抑制Aβ的聚集。最近,实验研究表明,生物ATP分子可防止Aβ原纤维形成纤颤。然而,ATP分子对Aβ纤颤的意义及其背后的机制仍然难以捉摸。我们使用ABER16SB,AMBER99SB-ILDN和AMBER-FB15力场对Aβ16-22肽进行了总计7.5μs的全原子分子动力学研究和8.82μs的伞状样品在清澈水中进行研究,以研究ATP在Aβ16-22肽上的作用。 Aβ16-22前原纤维的破坏。通过各种分析,例如二级结构分析,残基接触图,SASA和相互作用能,我们已经观察到,在存在ATP的情况下,Aβ16-22肽的聚集是非常不利的。而且,生物分子ATP通过氢键,π-π堆积和NH-π相互作用与Aβ16-22肽相互作用,这最终阻止了Aβ16-22肽的聚集。因此,我们假设ATP的缺乏可能会导致阿尔茨海默氏病(AD)。
更新日期:2019-12-20
中文翻译:

ATP控制Aβ16-22肽的聚集。
作为全长Aβ1-42的疏水核心区域的Aβ16-22肽的寡聚引起阿尔茨海默氏病(AD)。这种进行性神经退行性疾病影响全世界超过4400万人。但是,很少有合成的药物分子可用于抑制Aβ的聚集。最近,实验研究表明,生物ATP分子可防止Aβ原纤维形成纤颤。然而,ATP分子对Aβ纤颤的意义及其背后的机制仍然难以捉摸。我们使用ABER16SB,AMBER99SB-ILDN和AMBER-FB15力场对Aβ16-22肽进行了总计7.5μs的全原子分子动力学研究和8.82μs的伞状样品在清澈水中进行研究,以研究ATP在Aβ16-22肽上的作用。 Aβ16-22前原纤维的破坏。通过各种分析,例如二级结构分析,残基接触图,SASA和相互作用能,我们已经观察到,在存在ATP的情况下,Aβ16-22肽的聚集是非常不利的。而且,生物分子ATP通过氢键,π-π堆积和NH-π相互作用与Aβ16-22肽相互作用,这最终阻止了Aβ16-22肽的聚集。因此,我们假设ATP的缺乏可能会导致阿尔茨海默氏病(AD)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号