当前位置:
X-MOL 学术
›
Lancet Oncol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ribociclib plus letrozole versus chemotherapy for postmenopausal women with hormone receptor-positive, HER2-negative, luminal B breast cancer (CORALLEEN): an open-label, multicentre, randomised, phase 2 trial.
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-12-11 , DOI: 10.1016/s1470-2045(19)30786-7 Aleix Prat 1 , Cristina Saura 2 , Tomás Pascual 1 , Cristina Hernando 3 , Montserrat Muñoz 4 , Laia Paré 5 , Blanca González Farré 6 , Pedro L Fernández 7 , Patricia Galván 8 , Núria Chic 9 , Xavier González Farré 10 , Mafalda Oliveira 2 , Miguel Gil-Gil 11 , Miriam Arumi 12 , Neus Ferrer 13 , Alvaro Montaño 14 , Yann Izarzugaza 15 , Antonio Llombart-Cussac 16 , Raquel Bratos 17 , Santiago González Santiago 18 , Eduardo Martínez 19 , Sergio Hoyos 20 , Beatriz Rojas 21 , Juan Antonio Virizuela 22 , Vanesa Ortega 23 , Rafael López 24 , Pamela Céliz 5 , Eva Ciruelos 25 , Patricia Villagrasa 5 , Joaquín Gavilá 26
The Lancet Oncology ( IF 41.6 ) Pub Date : 2019-12-11 , DOI: 10.1016/s1470-2045(19)30786-7 Aleix Prat 1 , Cristina Saura 2 , Tomás Pascual 1 , Cristina Hernando 3 , Montserrat Muñoz 4 , Laia Paré 5 , Blanca González Farré 6 , Pedro L Fernández 7 , Patricia Galván 8 , Núria Chic 9 , Xavier González Farré 10 , Mafalda Oliveira 2 , Miguel Gil-Gil 11 , Miriam Arumi 12 , Neus Ferrer 13 , Alvaro Montaño 14 , Yann Izarzugaza 15 , Antonio Llombart-Cussac 16 , Raquel Bratos 17 , Santiago González Santiago 18 , Eduardo Martínez 19 , Sergio Hoyos 20 , Beatriz Rojas 21 , Juan Antonio Virizuela 22 , Vanesa Ortega 23 , Rafael López 24 , Pamela Céliz 5 , Eva Ciruelos 25 , Patricia Villagrasa 5 , Joaquín Gavilá 26
Affiliation
|
BACKGROUND
In hormone receptor-positive, HER2-negative early stage breast cancer, cyclin-dependent kinases 4 and 6 (CDK4/6) inhibition in combination with endocrine therapy could represent an alternative to multiagent chemotherapy. We aimed to evaluate the biological and clinical activity of neoadjuvant ribociclib plus letrozole in the luminal B subtype of early stage breast cancer.
METHODS
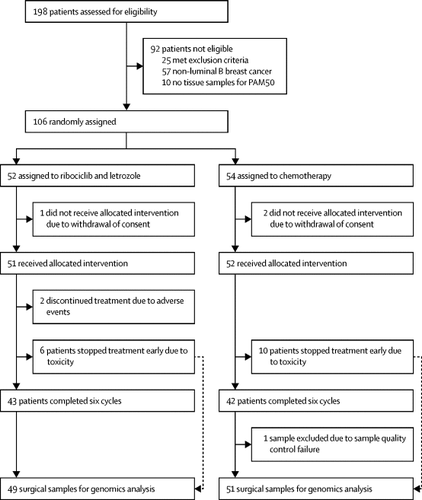
CORALLEEN is a parallel-arm, multicentre, randomised, open-label, phase 2 trial completed across 21 hospitals in Spain. We recruited postmenopausal women (≥18 years) with stage I-IIIA hormone receptor-positive, Eastern Cooperative Oncology Group Performance Status 0-1, HER2-negative breast cancer and luminal B by PAM50 with histologically confirmed, operable primary tumour size of at least 2 cm in diameter as measured by MRI. Patients were randomly assigned (1:1) using a web-based system and permuted blocks of 25 to receive either six 28-days cycles of ribociclib (oral 600 mg once daily for 3 weeks on, 1 week off) plus daily letrozole (oral 2·5 mg/day) or four cycles of doxorubicin (intravenous 60 mg/m2) and cyclophosphamide (intravenous 600 mg/m2) every 21 days followed by weekly paclitaxel (intravenous 80 mg/m2) for 12 weeks. The total duration of the neoadjuvant therapy was 24 weeks. Randomisation was stratified by tumour size and nodal involvement. Samples were prospectively collected at baseline (day 0), day 15, and surgery. The primary endpoint was to evaluate the proportion of patients with PAM50 low-risk-of-relapse (ROR) disease at surgery in the modified intention-to-treat population including all randomly assigned patients who received study drug and had a baseline and at least one post-baseline measurement of ROR score. The PAM50 ROR risk class integrated gene expression data, tumour size, and nodal status to define prognosis. This trial was registered at ClinicalTrials.gov, NCT03248427.
FINDINGS
Between July 27, 2017 to Dec 7, 2018, 106 patients were enrolled. At baseline, of the 106 patients, 92 (87%) patients had high ROR disease (44 [85%] of 52 in the ribociclib and letrozole group and 48 [89%] of 54 in the chemotherapy group) and 14 (13%) patients had intermediate-ROR disease (eight [15%] and six [11%]). Median follow-up was 200·0 days (IQR 191·2-206·0). At surgery, 23 (46·9%; 95% CI 32·5-61·7) of 49 patients in the ribociclib plus letrozole group and 24 (46·1%; 32·9-61·5) of 52 patients in the chemotherapy group were low-ROR. The most common grade 3-4 adverse events in the ribociclib plus letrozole group were neutropenia (22 [43%] of 51 patients) and elevated alanine aminotransferase concentrations (ten [20%]). The most common grade 3-4 adverse events in the chemotherapy group were neutropenia (31 [60%] of 52 patients) and febrile neutropenia (seven [13%]). No deaths were observed during the study in either group.
INTERPRETATION
Our results suggest that some patients with high-risk, early stage, hormone receptor-positive, HER2-negative breast cancer could achieve molecular downstaging of their disease with CDK4/6 inhibitor and endocrine therapy.
FUNDING
Novartis, Nanostring, Breast Cancer Research Foundation-AACR Career Development Award.
中文翻译:

Ribociclib联合来曲唑与化疗联合用于激素受体阳性,HER2阴性,管腔B乳腺癌(CORALLEEN)的绝经后妇女:一项开放性,多中心,随机,2期试验。
背景技术在激素受体阳性,HER2阴性的早期乳腺癌中,细胞周期蛋白依赖性激酶4和6(CDK4 / 6)抑制与内分泌治疗相结合可以代表多药化疗的替代方案。我们旨在评估在早期乳腺癌的管腔B亚型中新辅助的ribociclib加来曲唑的生物学和临床活性。方法CORALLEEN是一项在西班牙的21家医院中完成的一项平行,多中心,随机,开放标签的2期临床试验。我们招募了绝经后妇女(≥18岁),其I-IIIA期激素受体阳性,东部合作肿瘤小组表现为0-1,HER2阴性乳腺癌和PAM50管腔B均经组织学证实,可手术的原发肿瘤大小至少为通过MRI测量的直径为2厘米。患者被随机分配(1:1)使用基于网络的系统和25个置换排列的区块,以接受六个28天周期的核糖嘧啶核苷(口服600毫克,每天一次,连续3周,休假1周),再加上每日来曲唑(口服2·5毫克/天)或每21天四个周期的阿霉素(静脉注射60 mg / m2)和环磷酰胺(静脉注射600 mg / m2),然后每周一次紫杉醇(静脉注射80 mg / m2),持续12周。新辅助治疗的总持续时间为24周。肿瘤大小和淋巴结受累将随机分组。前瞻性地在基线(第0天),第15天和手术时收集样品。主要终点是评估改良意向治疗人群中手术时患有PAM50低复发风险(ROR)疾病的患者比例,包括所有随机分配的接受研究药物且基线且至少基线后ROR评分的一种测量。PAM50 ROR风险类别整合了基因表达数据,肿瘤大小和淋巴结状态以定义预后。该试验已在ClinicalTrials.gov上注册,NCT03248427。结果在2017年7月27日至2018年12月7日之间,共招募了106名患者。基线时,在106例患者中,有92例(87%)患ROR病(核糖体和来曲唑组52例,占44 [85%],化疗组54例,占48 [89%]),14例(13% )患者患有中度ROR疾病(8例[15%]和6例[11%])。中位随访时间为200·0天(IQR 191·2-206·0)。手术时,利比昔布加来曲唑组49例患者中有23例(46·9%; 95%CI 32·5-61·7)和52例中52例中24例(46·1%; 32·9-61·5)化疗组为低ROR。核糖体联合来曲唑组中最常见的3-4级不良事件是中性粒细胞减少症(51名患者中的22名[43%])和丙氨酸转氨酶浓度升高(十名[20%])。化疗组中最常见的3-4级不良事件为中性粒细胞减少症(52名患者中的31名[60%])和高热性中性粒细胞减少症(七名[13%])。在研究中,两组均未观察到死亡。解释我们的结果表明,某些高危,早期,激素受体阳性,HER2阴性乳腺癌患者可以通过CDK4 / 6抑制剂和内分泌治疗使疾病的分子水平降低。资助诺华,纳米线,
更新日期:2020-01-04
中文翻译:

Ribociclib联合来曲唑与化疗联合用于激素受体阳性,HER2阴性,管腔B乳腺癌(CORALLEEN)的绝经后妇女:一项开放性,多中心,随机,2期试验。
背景技术在激素受体阳性,HER2阴性的早期乳腺癌中,细胞周期蛋白依赖性激酶4和6(CDK4 / 6)抑制与内分泌治疗相结合可以代表多药化疗的替代方案。我们旨在评估在早期乳腺癌的管腔B亚型中新辅助的ribociclib加来曲唑的生物学和临床活性。方法CORALLEEN是一项在西班牙的21家医院中完成的一项平行,多中心,随机,开放标签的2期临床试验。我们招募了绝经后妇女(≥18岁),其I-IIIA期激素受体阳性,东部合作肿瘤小组表现为0-1,HER2阴性乳腺癌和PAM50管腔B均经组织学证实,可手术的原发肿瘤大小至少为通过MRI测量的直径为2厘米。患者被随机分配(1:1)使用基于网络的系统和25个置换排列的区块,以接受六个28天周期的核糖嘧啶核苷(口服600毫克,每天一次,连续3周,休假1周),再加上每日来曲唑(口服2·5毫克/天)或每21天四个周期的阿霉素(静脉注射60 mg / m2)和环磷酰胺(静脉注射600 mg / m2),然后每周一次紫杉醇(静脉注射80 mg / m2),持续12周。新辅助治疗的总持续时间为24周。肿瘤大小和淋巴结受累将随机分组。前瞻性地在基线(第0天),第15天和手术时收集样品。主要终点是评估改良意向治疗人群中手术时患有PAM50低复发风险(ROR)疾病的患者比例,包括所有随机分配的接受研究药物且基线且至少基线后ROR评分的一种测量。PAM50 ROR风险类别整合了基因表达数据,肿瘤大小和淋巴结状态以定义预后。该试验已在ClinicalTrials.gov上注册,NCT03248427。结果在2017年7月27日至2018年12月7日之间,共招募了106名患者。基线时,在106例患者中,有92例(87%)患ROR病(核糖体和来曲唑组52例,占44 [85%],化疗组54例,占48 [89%]),14例(13% )患者患有中度ROR疾病(8例[15%]和6例[11%])。中位随访时间为200·0天(IQR 191·2-206·0)。手术时,利比昔布加来曲唑组49例患者中有23例(46·9%; 95%CI 32·5-61·7)和52例中52例中24例(46·1%; 32·9-61·5)化疗组为低ROR。核糖体联合来曲唑组中最常见的3-4级不良事件是中性粒细胞减少症(51名患者中的22名[43%])和丙氨酸转氨酶浓度升高(十名[20%])。化疗组中最常见的3-4级不良事件为中性粒细胞减少症(52名患者中的31名[60%])和高热性中性粒细胞减少症(七名[13%])。在研究中,两组均未观察到死亡。解释我们的结果表明,某些高危,早期,激素受体阳性,HER2阴性乳腺癌患者可以通过CDK4 / 6抑制剂和内分泌治疗使疾病的分子水平降低。资助诺华,纳米线,











































 京公网安备 11010802027423号
京公网安备 11010802027423号