Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oseltamivir plus usual care versus usual care for influenza-like illness in primary care: an open-label, pragmatic, randomised controlled trial.
The Lancet ( IF 98.4 ) Pub Date : 2019-12-12 , DOI: 10.1016/s0140-6736(19)32982-4 Christopher C Butler 1 , Alike W van der Velden 2 , Emily Bongard 1 , Benjamin R Saville 3 , Jane Holmes 4 , Samuel Coenen 5 , Johanna Cook 1 , Nick A Francis 6 , Roger J Lewis 7 , Maciek Godycki-Cwirko 8 , Carl Llor 9 , Sławomir Chlabicz 10 , Christos Lionis 11 , Bohumil Seifert 12 , Pär-Daniel Sundvall 13 , Annelies Colliers 5 , Rune Aabenhus 14 , Lars Bjerrum 14 , Nicolay Jonassen Harbin 15 , Morten Lindbæk 15 , Dominik Glinz 16 , Heiner C Bucher 16 , Bernadett Kovács 17 , Ruta Radzeviciene Jurgute 18 , Pia Touboul Lundgren 19 , Paul Little 6 , Andrew W Murphy 20 , An De Sutter 21 , Peter Openshaw 22 , Menno D de Jong 23 , Jason T Connor 24 , Veerle Matheeussen 25 , Margareta Ieven 25 , Herman Goossens 25 , Theo J Verheij 2
The Lancet ( IF 98.4 ) Pub Date : 2019-12-12 , DOI: 10.1016/s0140-6736(19)32982-4 Christopher C Butler 1 , Alike W van der Velden 2 , Emily Bongard 1 , Benjamin R Saville 3 , Jane Holmes 4 , Samuel Coenen 5 , Johanna Cook 1 , Nick A Francis 6 , Roger J Lewis 7 , Maciek Godycki-Cwirko 8 , Carl Llor 9 , Sławomir Chlabicz 10 , Christos Lionis 11 , Bohumil Seifert 12 , Pär-Daniel Sundvall 13 , Annelies Colliers 5 , Rune Aabenhus 14 , Lars Bjerrum 14 , Nicolay Jonassen Harbin 15 , Morten Lindbæk 15 , Dominik Glinz 16 , Heiner C Bucher 16 , Bernadett Kovács 17 , Ruta Radzeviciene Jurgute 18 , Pia Touboul Lundgren 19 , Paul Little 6 , Andrew W Murphy 20 , An De Sutter 21 , Peter Openshaw 22 , Menno D de Jong 23 , Jason T Connor 24 , Veerle Matheeussen 25 , Margareta Ieven 25 , Herman Goossens 25 , Theo J Verheij 2
Affiliation
|
BACKGROUND
Antivirals are infrequently prescribed in European primary care for influenza-like illness, mostly because of perceived ineffectiveness in real world primary care and because individuals who will especially benefit have not been identified in independent trials. We aimed to determine whether adding antiviral treatment to usual primary care for patients with influenza-like illness reduces time to recovery overall and in key subgroups.
METHODS
We did an open-label, pragmatic, adaptive, randomised controlled trial of adding oseltamivir to usual care in patients aged 1 year and older presenting with influenza-like illness in primary care. The primary endpoint was time to recovery, defined as return to usual activities, with fever, headache, and muscle ache minor or absent. The trial was designed and powered to assess oseltamivir benefit overall and in 36 prespecified subgroups defined by age, comorbidity, previous symptom duration, and symptom severity, using a Bayesian piece-wise exponential primary analysis model. The trial is registered with the ISRCTN Registry, number ISRCTN 27908921.
FINDINGS
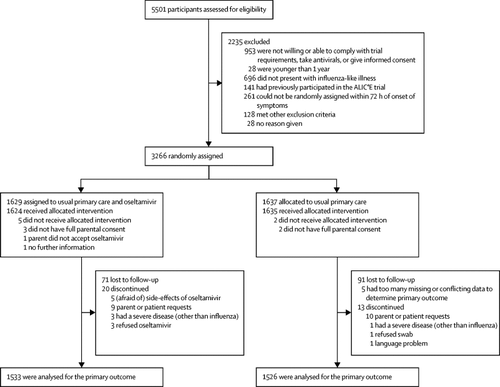
Between Jan 15, 2016, and April 12, 2018, we recruited 3266 participants in 15 European countries during three seasonal influenza seasons, allocated 1629 to usual care plus oseltamivir and 1637 to usual care, and ascertained the primary outcome in 1533 (94%) and 1526 (93%). 1590 (52%) of 3059 participants had PCR-confirmed influenza infection. Time to recovery was shorter in participants randomly assigned to oseltamivir (hazard ratio 1·29, 95% Bayesian credible interval [BCrI] 1·20-1·39) overall and in 30 of the 36 prespecified subgroups, with estimated hazard ratios ranging from 1·13 to 1·72. The estimated absolute mean benefit from oseltamivir was 1·02 days (95% [BCrI] 0·74-1·31) overall, and in the prespecified subgroups, ranged from 0·70 (95% BCrI 0·30-1·20) in patients younger than 12 years, with less severe symptoms, no comorbidities, and shorter previous illness duration to 3·20 (95% BCrI 1·00-5·50) in patients aged 65 years or older who had more severe illness, comorbidities, and longer previous illness duration. Regarding harms, an increased burden of vomiting or nausea was observed in the oseltamivir group.
INTERPRETATION
Primary care patients with influenza-like illness treated with oseltamivir recovered one day sooner on average than those managed by usual care alone. Older, sicker patients with comorbidities and longer previous symptom duration recovered 2-3 days sooner.
FUNDING
European Commission's Seventh Framework Programme.
中文翻译:

奥司他韦加常规护理与初级保健中流感样疾病的常规护理:一项开放标签、务实、随机对照试验。
背景技术在欧洲初级保健中很少针对流感样疾病开出抗病毒药物,主要是因为在现实世界初级保健中被认为无效,并且因为在独立试验中尚未确定特别受益的个体。我们的目的是确定在流感样疾病患者的常规初级保健中增加抗病毒治疗是否会缩短整体和关键亚组的康复时间。方法 我们进行了一项开放标签、务实、适应性、随机对照试验,将奥司他韦添加到初级保健中出现流感样疾病的 1 岁及以上患者的常规护理中。主要终点是恢复时间,定义为恢复正常活动,伴有发烧、头痛和轻微或不出现肌肉疼痛。该试验的设计和动力是使用贝叶斯分段指数主要分析模型,评估奥司他韦的总体获益以及根据年龄、合并症、既往症状持续时间和症状严重程度定义的 36 个预先指定的亚组中的获益。该试验已在 ISRCTN 登记处注册,编号 ISRCTN 27908921。 结果 在 2016 年 1 月 15 日至 2018 年 4 月 12 日期间,我们在三个季节性流感季节期间在 15 个欧洲国家招募了 3266 名参与者,其中 1629 人接受常规治疗加奥司他韦,1637 人接受常规治疗加奥司他韦治疗。常规护理,并在 1533 年(94%)和 1526 年(93%)确定了主要结局。 3059 名参与者中有 1590 名(52%)患有 PCR 确诊的流感感染。总体而言,随机分配到奥司他韦组的参与者(风险比为 1·29,95% 贝叶斯可信区间 [BCrI] 1·20-1·39)以及 36 个预先指定的亚组中的 30 个亚组的恢复时间较短,估计的风险比范围为1·13 至 1·72。 奥司他韦的估计绝对平均获益总体为 1·02 天 (95% [BCrI] 0·74-1·31),在预先指定的亚组中,范围为 0·70 (95% BCrI 0·30-1·20) ) 年龄小于 12 岁、症状较轻、无合并症且既往病程较短至 3·20 (95% BCrI 1·00-5·50) 的患者;年龄在 65 岁或以上且病情较严重的患者,合并症和较长的既往病程。关于危害,奥司他韦组观察到呕吐或恶心负担增加。解释 接受奥司他韦治疗的患有流感样疾病的初级保健患者比仅接受常规护理的患者平均早一天康复。患有合并症且既往症状持续时间较长的老年、病情较重的患者康复时间会提前 2-3 天。资助欧盟委员会的第七个框架计划。
更新日期:2020-01-04
中文翻译:

奥司他韦加常规护理与初级保健中流感样疾病的常规护理:一项开放标签、务实、随机对照试验。
背景技术在欧洲初级保健中很少针对流感样疾病开出抗病毒药物,主要是因为在现实世界初级保健中被认为无效,并且因为在独立试验中尚未确定特别受益的个体。我们的目的是确定在流感样疾病患者的常规初级保健中增加抗病毒治疗是否会缩短整体和关键亚组的康复时间。方法 我们进行了一项开放标签、务实、适应性、随机对照试验,将奥司他韦添加到初级保健中出现流感样疾病的 1 岁及以上患者的常规护理中。主要终点是恢复时间,定义为恢复正常活动,伴有发烧、头痛和轻微或不出现肌肉疼痛。该试验的设计和动力是使用贝叶斯分段指数主要分析模型,评估奥司他韦的总体获益以及根据年龄、合并症、既往症状持续时间和症状严重程度定义的 36 个预先指定的亚组中的获益。该试验已在 ISRCTN 登记处注册,编号 ISRCTN 27908921。 结果 在 2016 年 1 月 15 日至 2018 年 4 月 12 日期间,我们在三个季节性流感季节期间在 15 个欧洲国家招募了 3266 名参与者,其中 1629 人接受常规治疗加奥司他韦,1637 人接受常规治疗加奥司他韦治疗。常规护理,并在 1533 年(94%)和 1526 年(93%)确定了主要结局。 3059 名参与者中有 1590 名(52%)患有 PCR 确诊的流感感染。总体而言,随机分配到奥司他韦组的参与者(风险比为 1·29,95% 贝叶斯可信区间 [BCrI] 1·20-1·39)以及 36 个预先指定的亚组中的 30 个亚组的恢复时间较短,估计的风险比范围为1·13 至 1·72。 奥司他韦的估计绝对平均获益总体为 1·02 天 (95% [BCrI] 0·74-1·31),在预先指定的亚组中,范围为 0·70 (95% BCrI 0·30-1·20) ) 年龄小于 12 岁、症状较轻、无合并症且既往病程较短至 3·20 (95% BCrI 1·00-5·50) 的患者;年龄在 65 岁或以上且病情较严重的患者,合并症和较长的既往病程。关于危害,奥司他韦组观察到呕吐或恶心负担增加。解释 接受奥司他韦治疗的患有流感样疾病的初级保健患者比仅接受常规护理的患者平均早一天康复。患有合并症且既往症状持续时间较长的老年、病情较重的患者康复时间会提前 2-3 天。资助欧盟委员会的第七个框架计划。











































 京公网安备 11010802027423号
京公网安备 11010802027423号