Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Use of anastrozole for breast cancer prevention (IBIS-II): long-term results of a randomised controlled trial.
The Lancet ( IF 168.9 ) Pub Date : 2019-12-12 , DOI: 10.1016/s0140-6736(19)32955-1 Jack Cuzick 1 , Ivana Sestak 1 , John F Forbes 2 , Mitch Dowsett 3 , Simon Cawthorn 4 , Robert E Mansel 5 , Sibylle Loibl 6 , Bernardo Bonanni 7 , D Gareth Evans 8 , Anthony Howell 8 ,
The Lancet ( IF 168.9 ) Pub Date : 2019-12-12 , DOI: 10.1016/s0140-6736(19)32955-1 Jack Cuzick 1 , Ivana Sestak 1 , John F Forbes 2 , Mitch Dowsett 3 , Simon Cawthorn 4 , Robert E Mansel 5 , Sibylle Loibl 6 , Bernardo Bonanni 7 , D Gareth Evans 8 , Anthony Howell 8 ,
Affiliation
|
BACKGROUND
Two large clinical trials have shown a reduced rate of breast cancer development in high-risk women in the initial 5 years of follow-up after use of aromatase inhibitors (MAP.3 and International Breast Cancer Intervention Study II [IBIS-II]). Here, we report blinded long-term follow-up results for the IBIS-II trial, which compared anastrozole with placebo, with the objective of determining the efficacy of anastrozole for preventing breast cancer (both invasive and ductal carcinoma in situ) in the post-treatment period.
METHODS
IBIS-II is an international, randomised, double-blind, placebo-controlled trial. Postmenopausal women at increased risk of developing breast cancer were recruited and were randomly assigned (1:1) to either anastrozole (1 mg per day, oral) or matching placebo daily for 5 years. After treatment completion, women were followed on a yearly basis to collect data on breast cancer incidence, death, other cancers, and major adverse events (cardiovascular events and fractures). The primary outcome was all breast cancer.
FINDINGS
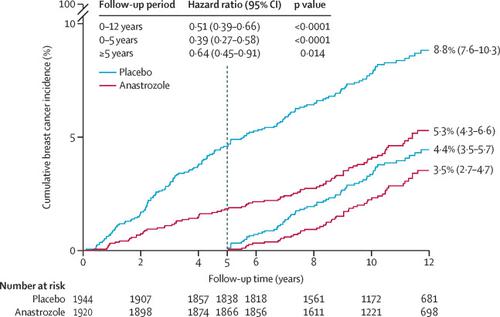
3864 women were recruited between Feb 2, 2003, and Jan 31, 2012. 1920 women were randomly assigned to 5 years anastrozole and 1944 to placebo. After a median follow-up of 131 months (IQR 105-156), a 49% reduction in breast cancer was observed for anastrozole (85 vs 165 cases, hazard ratio [HR] 0·51, 95% CI 0·39-0·66, p<0·0001). The reduction was larger in the first 5 years (35 vs 89, 0·39, 0·27-0·58, p<0·0001), but still significant after 5 years (50 vs 76 new cases, 0·64, 0·45-0·91, p=0·014), and not significantly different from the first 5 years (p=0·087). Invasive oestrogen receptor-positive breast cancer was reduced by 54% (HR 0·46, 95% CI 0·33-0·65, p<0·0001), with a continued significant effect in the period after treatment. A 59% reduction in ductal carcinoma in situ was observed (0·41, 0·22-0·79, p=0·0081), especially in participants known to be oestrogen receptor-positive (0·22, 0·78-0·65, p<0·0001). No significant difference in deaths was observed overall (69 vs 70, HR 0·96, 95% CI 0·69-1·34, p=0·82) or for breast cancer (two anastrozole vs three placebo). A significant decrease in non-breast cancers was observed for anastrozole (147 vs 200, odds ratio 0·72, 95% CI 0·57-0·91, p=0·0042), owing primarily to non-melanoma skin cancer. No excess of fractures or cardiovascular disease was observed.
INTERPRETATION
This analysis has identified a significant continuing reduction in breast cancer with anastrozole in the post-treatment follow-up period, with no evidence of new late side-effects. Further follow-up is needed to assess the effect on breast cancer mortality.
FUNDING
Cancer Research UK, the National Health and Medical Research Council Australia, Breast Cancer Research Foundation, Sanofi Aventis, and AstraZeneca.
中文翻译:

使用阿那曲唑预防乳腺癌(IBIS-II):一项随机对照试验的长期结果。
背景两项大型临床试验显示,在使用芳香酶抑制剂后的最初5年随访中,高危女性乳腺癌的发生率降低(MAP.3和国际乳腺癌干预研究II [IBIS-II])。 。在这里,我们报道了IBIS-II试验的盲目的长期随访结果,该试验将阿那曲唑与安慰剂进行了比较,目的是确定阿那曲唑在术后预防乳腺癌(侵袭性和导管原位癌)的功效。治疗期。方法IBIS-II是一项国际,随机,双盲,安慰剂对照试验。招募绝经后妇女,罹患乳腺癌的风险增加,并随机分配(1:1)服用阿那曲唑(每天1 mg,口服)或每天匹配安慰剂,持续5年。治疗结束后 每年对女性进行随访,以收集有关乳腺癌发生率,死亡,其他癌症和主要不良事件(心血管事件和骨折)的数据。主要结局是所有乳腺癌。结果2003年2月2日至2012年1月31日之间招募了3864名女性。将1920名女性随机分配至5年的阿那曲唑和1944年的安慰剂患者。经过131个月的中位随访(IQR 105-156),阿那曲唑的乳腺癌发生率降低了49%(85比165例,危险比[HR] 0·51、95%CI 0·39-0 ·66,p <0·0001)。在开始的5年中减少幅度较大(35 vs 89,0·39,0·27-0·58,p <0·0001),但在5年后仍然显着(50 vs 76新病例,0·64, 0·45-0·91,p = 0·014),与前5年无显着差异(p = 0·087)。侵袭性雌激素受体阳性的乳腺癌减少了54%(HR 0·46,95%CI 0·33-0·65,p <0·0001),在治疗后持续显着效果。观察到导管癌原位减少59%(0·41、0·22-0·79,p = 0·0081),尤其是在已知雌激素受体阳性的参与者中(0·22、0·78- 0·65,p <0·0001)。总体上(62%vs 70,HR 0·96,95%CI 0·69-1·34,p = 0·82)或乳腺癌(两个阿那曲唑对三个安慰剂)的死亡无显着差异。观察到阿那曲唑的非乳腺癌发生率显着下降(147比200,优势比0·72,95%CI 0·57-0·91,p = 0·0042),这主要归因于非黑素瘤皮肤癌。没有观察到过多的骨折或心血管疾病。解释这项分析已确定阿那曲唑在治疗后的随访期内乳腺癌的持续显着减少,没有新的晚期副作用的证据。需要进一步的随访以评估对乳腺癌死亡率的影响。英国癌症研究基金会,澳大利亚国家卫生与医学研究委员会,乳腺癌研究基金会,赛诺菲安万特和阿斯利康。
更新日期:2020-01-10
中文翻译:

使用阿那曲唑预防乳腺癌(IBIS-II):一项随机对照试验的长期结果。
背景两项大型临床试验显示,在使用芳香酶抑制剂后的最初5年随访中,高危女性乳腺癌的发生率降低(MAP.3和国际乳腺癌干预研究II [IBIS-II])。 。在这里,我们报道了IBIS-II试验的盲目的长期随访结果,该试验将阿那曲唑与安慰剂进行了比较,目的是确定阿那曲唑在术后预防乳腺癌(侵袭性和导管原位癌)的功效。治疗期。方法IBIS-II是一项国际,随机,双盲,安慰剂对照试验。招募绝经后妇女,罹患乳腺癌的风险增加,并随机分配(1:1)服用阿那曲唑(每天1 mg,口服)或每天匹配安慰剂,持续5年。治疗结束后 每年对女性进行随访,以收集有关乳腺癌发生率,死亡,其他癌症和主要不良事件(心血管事件和骨折)的数据。主要结局是所有乳腺癌。结果2003年2月2日至2012年1月31日之间招募了3864名女性。将1920名女性随机分配至5年的阿那曲唑和1944年的安慰剂患者。经过131个月的中位随访(IQR 105-156),阿那曲唑的乳腺癌发生率降低了49%(85比165例,危险比[HR] 0·51、95%CI 0·39-0 ·66,p <0·0001)。在开始的5年中减少幅度较大(35 vs 89,0·39,0·27-0·58,p <0·0001),但在5年后仍然显着(50 vs 76新病例,0·64, 0·45-0·91,p = 0·014),与前5年无显着差异(p = 0·087)。侵袭性雌激素受体阳性的乳腺癌减少了54%(HR 0·46,95%CI 0·33-0·65,p <0·0001),在治疗后持续显着效果。观察到导管癌原位减少59%(0·41、0·22-0·79,p = 0·0081),尤其是在已知雌激素受体阳性的参与者中(0·22、0·78- 0·65,p <0·0001)。总体上(62%vs 70,HR 0·96,95%CI 0·69-1·34,p = 0·82)或乳腺癌(两个阿那曲唑对三个安慰剂)的死亡无显着差异。观察到阿那曲唑的非乳腺癌发生率显着下降(147比200,优势比0·72,95%CI 0·57-0·91,p = 0·0042),这主要归因于非黑素瘤皮肤癌。没有观察到过多的骨折或心血管疾病。解释这项分析已确定阿那曲唑在治疗后的随访期内乳腺癌的持续显着减少,没有新的晚期副作用的证据。需要进一步的随访以评估对乳腺癌死亡率的影响。英国癌症研究基金会,澳大利亚国家卫生与医学研究委员会,乳腺癌研究基金会,赛诺菲安万特和阿斯利康。



























 京公网安备 11010802027423号
京公网安备 11010802027423号