当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A New Redox Mediator (Cupric‐Neocuproine Complex)−Modified Pencil Graphite Electrode for the Electrocatalytic Oxidation of H2O2: A Flow Injection Amperometric Sensor
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-01-07 , DOI: 10.1002/celc.201901765 Gamze Emir 1 , Yusuf Dilgin 1 , Reşat Apak 2
ChemElectroChem ( IF 3.5 ) Pub Date : 2020-01-07 , DOI: 10.1002/celc.201901765 Gamze Emir 1 , Yusuf Dilgin 1 , Reşat Apak 2
Affiliation
|
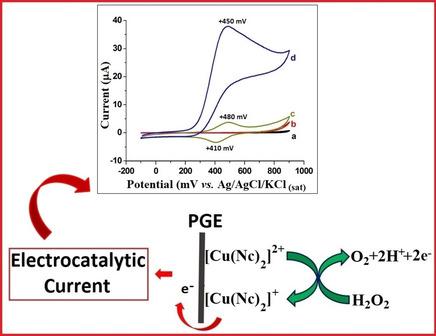
This paper introduces the cupric‐neocuproine complex ([Cu(Nc)2]2+) as a new redox mediator for the electrocatalytic oxidation of H2O2. For this purpose, [Cu(Nc)2]2+ was modified onto a disposable pencil graphite electrode (PGE) surface by using Nafion (Nf) as a cation‐exchange membrane for the effective adsorption of complex. The modified electrode was prepared by immersing Nf‐adsorbed PGE into [Cu(Nc)2]2+ complex solution (0.40 mM of Cu2+ and 0.80 mM of Nc prepared at pH 4.76 in 0.10 M acetate buffer). SEM images and EDX spectra of electrodes were presented for the elucidation of their surface morphologies. Cyclic voltammetric results showed that H2O2 was electrocatalytically oxidized at [Cu(Nc)2]2+/Nf/PGE, because both the increase in current and the potential shift to a more negative direction were observed for oxidation of H2O2 at [Cu(Nc)2]2+/Nf/PGE in comparison to bare PGE. Then, the electrocatalytic activity of [Cu(Nc)2]2+/Nf/PGE toward H2O2 oxidation was utilized for amperometric determination of H2O2 in flow injection analysis (FIA) system. Flow injection (FI) amperometric studies showed two linear calibration ranges (1.0‐1000.0 μM (R2=0.9958) and 1000.0‐10000.0 μM (R2=0.9902) with a detection limit of 0.4 μM H2O2 which is considered good for electroanalytical determinations, especially for electrocatalytic oxidation. To test the applicability of the developed FI amperometric hydrogen peroxide sensor, H2O2 contents of some relevant samples were determined.
中文翻译:

一种新的氧化还原介体(铜-新鸟嘌呤配合物)修饰的铅笔状石墨电极用于H2O2的电催化氧化:流动注射安培传感器
本文介绍了铜-新星嘌呤配合物([Cu(Nc)2 ] 2+)作为H 2 O 2电催化氧化的新型氧化还原介体。为此,通过使用Nafion(Nf)作为阳离子交换膜来有效地吸附复合物,将[Cu(Nc)2 ] 2+改性到了一次性铅笔石墨电极(PGE)的表面上。通过将吸附Nf的PGE浸入[Cu(Nc)2 ] 2+复合溶液(0.40 mM Cu 2+在0.10 M乙酸盐缓冲液中在pH 4.76下制备的0.80 mM Nc)。给出了电极的SEM图像和EDX光谱,以阐明其表面形态。循环伏安法结果表明,H 2 O 2在[Cu(Nc)2 ] 2+ / Nf / PGE处被电催化氧化,因为观察到电流的增加和电势向更负的方向移动对H 2 O的氧化2在[铜(NC)2 ] 2+ / NF / PGE相比于裸PGE。然后,[Cu(Nc)2 ] 2+ / Nf / PGE对H 2 O 2的电催化活性氧化用于流动注射分析(FIA)系统中的安培法测定H 2 O 2。流动注射(FI)安培法研究显示两个线性校准范围(1.0-1000.0μM (R 2 = 0.9958)和1000.0-10000.0μM(R 2 = 0.9902),检出限为0.4μMH 2 O 2,被认为对为了分析开发的FI安培型过氧化氢传感器的适用性,对一些相关样品中的H 2 O 2含量进行了测定。
更新日期:2020-01-07
中文翻译:

一种新的氧化还原介体(铜-新鸟嘌呤配合物)修饰的铅笔状石墨电极用于H2O2的电催化氧化:流动注射安培传感器
本文介绍了铜-新星嘌呤配合物([Cu(Nc)2 ] 2+)作为H 2 O 2电催化氧化的新型氧化还原介体。为此,通过使用Nafion(Nf)作为阳离子交换膜来有效地吸附复合物,将[Cu(Nc)2 ] 2+改性到了一次性铅笔石墨电极(PGE)的表面上。通过将吸附Nf的PGE浸入[Cu(Nc)2 ] 2+复合溶液(0.40 mM Cu 2+在0.10 M乙酸盐缓冲液中在pH 4.76下制备的0.80 mM Nc)。给出了电极的SEM图像和EDX光谱,以阐明其表面形态。循环伏安法结果表明,H 2 O 2在[Cu(Nc)2 ] 2+ / Nf / PGE处被电催化氧化,因为观察到电流的增加和电势向更负的方向移动对H 2 O的氧化2在[铜(NC)2 ] 2+ / NF / PGE相比于裸PGE。然后,[Cu(Nc)2 ] 2+ / Nf / PGE对H 2 O 2的电催化活性氧化用于流动注射分析(FIA)系统中的安培法测定H 2 O 2。流动注射(FI)安培法研究显示两个线性校准范围(1.0-1000.0μM (R 2 = 0.9958)和1000.0-10000.0μM(R 2 = 0.9902),检出限为0.4μMH 2 O 2,被认为对为了分析开发的FI安培型过氧化氢传感器的适用性,对一些相关样品中的H 2 O 2含量进行了测定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号