Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineered Cell-Derived Microparticles Bi2Se3/DOX@MPs for Imaging Guided Synergistic Photothermal/Low-Dose Chemotherapy of Cancer.
Advanced Science ( IF 14.3 ) Pub Date : 2019-12-12 , DOI: 10.1002/advs.201901293 Dongdong Wang 1 , Yuzhu Yao 1 , Junkai He 1 , Xiaoyan Zhong 1 , Basen Li 2 , Shiyu Rao 1 , Haiting Yu 1 , Shuaicheng He 1 , Xiaoyu Feng 1 , Tuo Xu 1 , Bin Yang 1 , Tuying Yong 1 , Lu Gan 1 , Jun Hu 1 , Xiangliang Yang 1
Advanced Science ( IF 14.3 ) Pub Date : 2019-12-12 , DOI: 10.1002/advs.201901293 Dongdong Wang 1 , Yuzhu Yao 1 , Junkai He 1 , Xiaoyan Zhong 1 , Basen Li 2 , Shiyu Rao 1 , Haiting Yu 1 , Shuaicheng He 1 , Xiaoyu Feng 1 , Tuo Xu 1 , Bin Yang 1 , Tuying Yong 1 , Lu Gan 1 , Jun Hu 1 , Xiangliang Yang 1
Affiliation
|
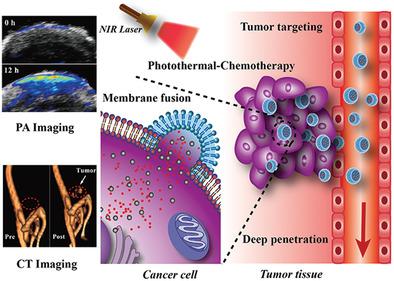
Cell-derived microparticles, which are recognized as nanosized phospholipid bilayer membrane vesicles, have exhibited great potential to serve as drug delivery systems in cancer therapy. However, for the purpose of comprehensive therapy, microparticles decorated with multiple therapeutic components are needed, but effective engineering strategies are limited and still remain enormous challenges. Herein, Bi2Se3 nanodots and doxorubicin hydrochloride (DOX) co-embedded tumor cell-derived microparticles (Bi2Se3/DOX@MPs) are successfully constructed through ultraviolet light irradiation-induced budding of parent cells which are preloaded with Bi2Se3 nanodots and DOX via electroporation. The multifunctional microparticles are obtained with high controllability and drug-loading capacity without unfavorable membrane surface destruction, maintaining their excellent intrinsic biological behaviors. Through membrane fusion cellular internalization, Bi2Se3/DOX@MPs show enhanced cellular internalization and deepened tumor penetration, resulting in extreme cell damage in vitro without considering endosomal escape. Because of their distinguished photothermal performance and tumor homing target capability, Bi2Se3/DOX@MPs exhibit admirable dual-modal imaging capacity and outstanding tumor suppression effect. Under 808 nm laser irradiation, intravenous injection of Bi2Se3/DOX@MPs into H22 tumor-bearing mice results in remarkably synergistic antitumor efficacy by combining photothermal therapy with low-dose chemotherapy in vivo. Furthermore, the negligible hemolytic activity, considerable metabolizability, and low systemic toxicity of Bi2Se3/DOX@MPs imply their distinguished biocompatibility and great potential for tumor theranostics.
中文翻译:

用于癌症成像引导协同光热/低剂量化疗的工程细胞衍生微粒 Bi2Se3/DOX@MP。
细胞衍生的微粒被认为是纳米级磷脂双层膜囊泡,在癌症治疗中作为药物输送系统表现出巨大的潜力。然而,为了综合治疗的目的,需要装饰有多种治疗成分的微粒,但有效的工程策略有限,仍然面临巨大的挑战。在此,通过紫外光照射诱导母细胞出芽,成功构建了 Bi2Se3 纳米点和盐酸阿霉素(DOX)共嵌入的肿瘤细胞源微粒(Bi2Se3/DOX@MPs),并通过电穿孔预载 Bi2Se3 纳米点和 DOX。获得的多功能微粒具有高可控性和载药量,且不会对膜表面造成不利的破坏,保持其优异的固有生物学行为。通过膜融合细胞内化,Bi2Se3/DOX@MPs 显示出增强的细胞内化和加深的肿瘤渗透,在不考虑内体逃逸的情况下导致体外极端的细胞损伤。由于其卓越的光热性能和肿瘤归巢能力,Bi2Se3/DOX@MPs表现出令人惊叹的双模态成像能力和突出的肿瘤抑制效果。在808 nm激光照射下,将Bi2Se3/DOX@MPs静脉注射到H22荷瘤小鼠体内,通过将光热疗法与低剂量化疗相结合,产生显着的协同抗肿瘤功效。此外,Bi2Se3/DOX@MPs 的可忽略的溶血活性、相当大的代谢性和较低的全身毒性意味着它们具有卓越的生物相容性和肿瘤治疗诊断的巨大潜力。
更新日期:2019-12-13
中文翻译:

用于癌症成像引导协同光热/低剂量化疗的工程细胞衍生微粒 Bi2Se3/DOX@MP。
细胞衍生的微粒被认为是纳米级磷脂双层膜囊泡,在癌症治疗中作为药物输送系统表现出巨大的潜力。然而,为了综合治疗的目的,需要装饰有多种治疗成分的微粒,但有效的工程策略有限,仍然面临巨大的挑战。在此,通过紫外光照射诱导母细胞出芽,成功构建了 Bi2Se3 纳米点和盐酸阿霉素(DOX)共嵌入的肿瘤细胞源微粒(Bi2Se3/DOX@MPs),并通过电穿孔预载 Bi2Se3 纳米点和 DOX。获得的多功能微粒具有高可控性和载药量,且不会对膜表面造成不利的破坏,保持其优异的固有生物学行为。通过膜融合细胞内化,Bi2Se3/DOX@MPs 显示出增强的细胞内化和加深的肿瘤渗透,在不考虑内体逃逸的情况下导致体外极端的细胞损伤。由于其卓越的光热性能和肿瘤归巢能力,Bi2Se3/DOX@MPs表现出令人惊叹的双模态成像能力和突出的肿瘤抑制效果。在808 nm激光照射下,将Bi2Se3/DOX@MPs静脉注射到H22荷瘤小鼠体内,通过将光热疗法与低剂量化疗相结合,产生显着的协同抗肿瘤功效。此外,Bi2Se3/DOX@MPs 的可忽略的溶血活性、相当大的代谢性和较低的全身毒性意味着它们具有卓越的生物相容性和肿瘤治疗诊断的巨大潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号