当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ag@Au Core-Shell Nanowires for Nearly 100 % CO2 -to-CO Electroreduction.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2019-12-27 , DOI: 10.1002/asia.201901550 Jinze Liu 1 , Yating Wang 2 , Hao Jiang 1, 2 , Haibo Jiang 2 , Xiaodong Zhou 1 , Yuhang Li 2 , Chunzhong Li 1, 2
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2019-12-27 , DOI: 10.1002/asia.201901550 Jinze Liu 1 , Yating Wang 2 , Hao Jiang 1, 2 , Haibo Jiang 2 , Xiaodong Zhou 1 , Yuhang Li 2 , Chunzhong Li 1, 2
Affiliation
|
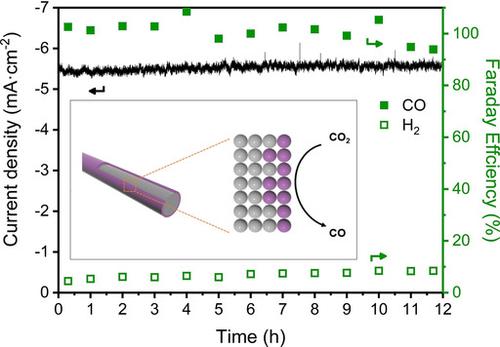
Electrochemical reduction of carbon dioxide (CO2 ) to CO is regarded as an efficient method to utilize the greenhouse gas CO2 , because the CO product can be further converted into high value-added chemicals via the Fisher-Tropsch process. Among all electrocatalysts used for CO2 -to-CO reduction, Au-based catalysts have been demonstrated to possess high selectivity, but their precious price limits their future large-scale applications. Thus, simultaneously achieving high selectivity and reasonable price is of great importance for the development of Au-based catalysts. Here, we report Ag@Au core-shell nanowires as electrocatalyst for CO2 reduction, in which a nanometer-thick Au film is uniformly deposited on the core Ag nanowire. Importantly, the Ag@Au catalyst with a relative low Au content can drive CO generation with nearly 100 % Faraday efficiency in 0.1 m KCl electrolyte at an overpotential of ca. -1.0 V. This high selectivity of CO2 reduction could be attributed to a suitable adsorption strength for the key intermediate on Au film together with the synergistic effects between the Au shell and Ag core and the strong interaction between CO2 and Cl- ions in the electrolyte, which may further pave the way for the development of high-efficiency electrocatalysts for CO2 reduction.
中文翻译:

Ag @ Au核壳纳米线可实现近100%的CO2到CO的电还原。
将二氧化碳(CO2)电化学还原为CO被认为是利用温室气体CO2的有效方法,因为该CO产品可以通过Fisher-Tropsch工艺进一步转化为高附加值的化学品。在用于CO2到CO还原的所有电催化剂中,金基催化剂已被证明具有很高的选择性,但其昂贵的价格限制了它们未来的大规模应用。因此,同时获得高选择性和合理的价格对于开发Au基催化剂非常重要。在这里,我们报道了Ag @ Au核壳纳米线作为还原CO2的电催化剂,其中纳米厚度的Au膜均匀地沉积在核心Ag纳米线上。重要的,Au含量相对较低的Ag @ Au催化剂可在0.1 m KCl的电解液中以约100的超电势驱动CO生成,并具有近100%的法拉第效率。-1.0V。CO2还原的高选择性归因于关键中间体在Au膜上的合适吸附强度,以及Au壳层与Ag核之间的协同作用以及电解质中CO2与Cl-离子之间的强相互作用,这可能会进一步为开发用于还原CO2的高效电催化剂铺平道路。
更新日期:2019-12-27
中文翻译:

Ag @ Au核壳纳米线可实现近100%的CO2到CO的电还原。
将二氧化碳(CO2)电化学还原为CO被认为是利用温室气体CO2的有效方法,因为该CO产品可以通过Fisher-Tropsch工艺进一步转化为高附加值的化学品。在用于CO2到CO还原的所有电催化剂中,金基催化剂已被证明具有很高的选择性,但其昂贵的价格限制了它们未来的大规模应用。因此,同时获得高选择性和合理的价格对于开发Au基催化剂非常重要。在这里,我们报道了Ag @ Au核壳纳米线作为还原CO2的电催化剂,其中纳米厚度的Au膜均匀地沉积在核心Ag纳米线上。重要的,Au含量相对较低的Ag @ Au催化剂可在0.1 m KCl的电解液中以约100的超电势驱动CO生成,并具有近100%的法拉第效率。-1.0V。CO2还原的高选择性归因于关键中间体在Au膜上的合适吸附强度,以及Au壳层与Ag核之间的协同作用以及电解质中CO2与Cl-离子之间的强相互作用,这可能会进一步为开发用于还原CO2的高效电催化剂铺平道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号