当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
LncRNA ODIR1 inhibits osteogenic differentiation of hUC-MSCs through the FBXO25/H2BK120ub/H3K4me3/OSX axis.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-12-11 , DOI: 10.1038/s41419-019-2148-2 Shiwei He 1, 2, 3 , Sheng Yang 4 , Yanru Zhang 1 , Xiaoling Li 1 , Dan Gao 1 , Yancheng Zhong 1 , Lihua Cao 1 , Haotian Ma 1 , Ying Liu 1 , Guiyuan Li 1 , Shuping Peng 1, 2, 3 , Cijun Shuai 5, 6
Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-12-11 , DOI: 10.1038/s41419-019-2148-2 Shiwei He 1, 2, 3 , Sheng Yang 4 , Yanru Zhang 1 , Xiaoling Li 1 , Dan Gao 1 , Yancheng Zhong 1 , Lihua Cao 1 , Haotian Ma 1 , Ying Liu 1 , Guiyuan Li 1 , Shuping Peng 1, 2, 3 , Cijun Shuai 5, 6
Affiliation
|
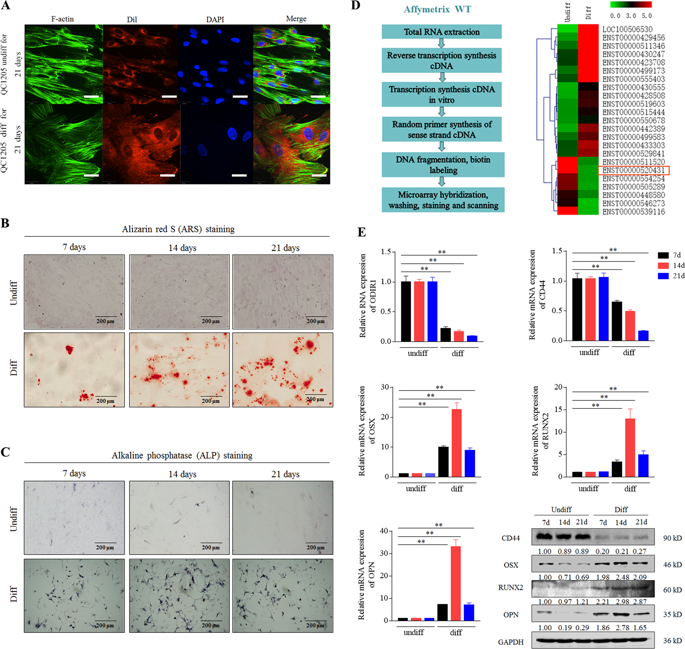
Long noncoding RNAs (lncRNAs) have been demonstrated to be important regulators during the osteogenic differentiation of mesenchymal stem cells (MSCs). We analyzed the lncRNA expression profile during osteogenic differentiation of human umbilical cord-derived mesenchymal stem cells (hUC-MSCs) and identified a significantly downregulated lncRNA RP11-527N22.2, named osteogenic differentiation inhibitory lncRNA 1, ODIR1. In hUC-MSCs, ODIR1 knockdown significantly promoted osteogenic differentiation, whereas overexpression inhibited osteogenic differentiation in vitro and in vivo. Mechanistically, ODIR1 interacts with F-box protein 25 (FBXO25) and facilitates the proteasome-dependent degradation of FBXO25 by recruiting Cullin 3 (CUL3). FBXO25 increases the mono-ubiquitination of H2BK120 (H2BK120ub) which subsequently promotes the trimethylation of H3K4 (H3K4me3). Both H2BK120ub and H3K4me3 form a loose chromatin structure, inducing the transcription of the key transcription factor osterix (OSX) and increasing the expression of the downstream osteoblast markers, osteocalcin (OCN), osteopontin (OPN), and alkaline phosphatase (ALP). In summary, ODIR1 acts as a key negative regulator during the osteogenic differentiation of hUC-MSCs through the FBXO25/H2BK120ub/H3K4me3/OSX axis, which may provide a novel understanding of lncRNAs that regulate the osteogenesis of MSCs and a potential therapeutic strategy for the regeneration of bone defects.
中文翻译:

LncRNA ODIR1 通过 FBXO25/H2BK120ub/H3K4me3/OSX 轴抑制 hUC-MSC 的成骨分化。
长非编码RNA(lncRNA)已被证明是间充质干细胞(MSC)成骨分化过程中的重要调节因子。我们分析了人脐带间充质干细胞(hUC-MSC)成骨分化过程中的lncRNA表达谱,发现了一个显着下调的lncRNA RP11-527N22.2,命名为成骨分化抑制lncRNA 1,ODIR1。在 hUC-MSC 中,ODIR1 敲低显着促进成骨分化,而过表达则抑制体外和体内成骨分化。从机制上讲,ODIR1 与 F-box 蛋白 25 (FBXO25) 相互作用,并通过招募 Cullin 3 (CUL3) 促进 FBXO25 的蛋白酶体依赖性降解。 FBXO25 增加 H2BK120 (H2BK120ub) 的单泛素化,随后促进 H3K4 (H3K4me3) 的三甲基化。 H2BK120ub 和 H3K4me3 均形成松散的染色质结构,诱导关键转录因子 osterix (OSX) 的转录,并增加下游成骨细胞标志物骨钙素 (OCN)、骨桥蛋白 (OPN) 和碱性磷酸酶 (ALP) 的表达。总之,ODIR1通过FBXO25/H2BK120ub/H3K4me3/OSX轴在hUC-MSCs成骨分化过程中充当关键的负调节因子,这可能为调节MSCs成骨的lncRNA提供新的理解,并为hUC-MSCs成骨分化提供潜在的治疗策略。骨缺损的再生。
更新日期:2019-12-11
中文翻译:

LncRNA ODIR1 通过 FBXO25/H2BK120ub/H3K4me3/OSX 轴抑制 hUC-MSC 的成骨分化。
长非编码RNA(lncRNA)已被证明是间充质干细胞(MSC)成骨分化过程中的重要调节因子。我们分析了人脐带间充质干细胞(hUC-MSC)成骨分化过程中的lncRNA表达谱,发现了一个显着下调的lncRNA RP11-527N22.2,命名为成骨分化抑制lncRNA 1,ODIR1。在 hUC-MSC 中,ODIR1 敲低显着促进成骨分化,而过表达则抑制体外和体内成骨分化。从机制上讲,ODIR1 与 F-box 蛋白 25 (FBXO25) 相互作用,并通过招募 Cullin 3 (CUL3) 促进 FBXO25 的蛋白酶体依赖性降解。 FBXO25 增加 H2BK120 (H2BK120ub) 的单泛素化,随后促进 H3K4 (H3K4me3) 的三甲基化。 H2BK120ub 和 H3K4me3 均形成松散的染色质结构,诱导关键转录因子 osterix (OSX) 的转录,并增加下游成骨细胞标志物骨钙素 (OCN)、骨桥蛋白 (OPN) 和碱性磷酸酶 (ALP) 的表达。总之,ODIR1通过FBXO25/H2BK120ub/H3K4me3/OSX轴在hUC-MSCs成骨分化过程中充当关键的负调节因子,这可能为调节MSCs成骨的lncRNA提供新的理解,并为hUC-MSCs成骨分化提供潜在的治疗策略。骨缺损的再生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号