当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Antiferromagnetic Alkali Metal Oxohydroxoferrates(III) with Correlated Hydrogen Bonding Systems.
ChemistryOpen ( IF 2.5 ) Pub Date : 2019-11-26 , DOI: 10.1002/open.201900287 Ralf Albrecht 1 , Jens Hunger 1 , Markus Hölzel 2 , Theresa Block 3 , Rainer Pöttgen 3 , Thomas Doert 1 , Michael Ruck 1, 4
ChemistryOpen ( IF 2.5 ) Pub Date : 2019-11-26 , DOI: 10.1002/open.201900287 Ralf Albrecht 1 , Jens Hunger 1 , Markus Hölzel 2 , Theresa Block 3 , Rainer Pöttgen 3 , Thomas Doert 1 , Michael Ruck 1, 4
Affiliation
|
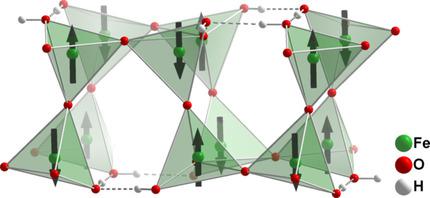
The oxohydroxoferrates(III) A2[Fe2O3(OH)2] (A=K, Rb, Cs) were synthesized under hydroflux conditions. Approximately equimolar mixtures of the alkali metal hydroxides and water were reacted with Fe(NO3)3 ⋅ 9H2O at about 200 °C. The product formation depends on the hydroxide concentration, therefore also other reaction products, such as KFeO2, K2−x[Fe4O7−x(OH)x] or α‐Fe2O3, are obtained. The crystal structures of the oxohydroxoferrates(III) A2[Fe2O3(OH)2] follow the same structural principle, yet differ in their layer stacking or/and their hydrogen bonding systems depending on A and temperature. In the resulting four different orthorhombic structure types, [FeO3OH]4− tetrahedra share their oxide corners to create folded  Fe2O3(OH)2]2− layers. The terminal hydroxide ligands form hydrogen bonds between and/or within the layers. The positions of the hydrogen atoms in these networks are correlated. The A+ cations are located between the folded anionic layers as well as in their trenches. Under reaction conditions, the potassium compound crystallizes in the space group Cmce (Pearson symbol oC88), showing a bimodal disorder of the hydrogen atoms in hydrogen bridges. In a virtually hysteresis‐less first‐order transition at 340(2) K, the structure slightly distorts into the room‐temperature modification with the subgroup Pbca (oP88), and the hydrogen atoms order. The rubidium and caesium compounds are isostructural to each other but not to the potassium compound, and are always obtained as mixtures of two modifications with space groups Cmce (oC88′) and Immb (oI88). Upon heating, the oxohydroxoferrates decompose into their anhydrides AFeO2 and water. The type of hydrogen bonding network influences the decomposition temperature, the structure and the morphology of the crystals. Despite the presence of iron(III), which was confirmed by 57Fe‐Mössbauer spectroscopy, K2[Fe2O3(OH)2] is diamagnetic in the investigated temperature range between 1.8 and 300 K. Neutron diffraction revealed strong antiferromagnetic coupling of the magnetic moments, which are inverted in neighboring tetrahedra.
Fe2O3(OH)2]2− layers. The terminal hydroxide ligands form hydrogen bonds between and/or within the layers. The positions of the hydrogen atoms in these networks are correlated. The A+ cations are located between the folded anionic layers as well as in their trenches. Under reaction conditions, the potassium compound crystallizes in the space group Cmce (Pearson symbol oC88), showing a bimodal disorder of the hydrogen atoms in hydrogen bridges. In a virtually hysteresis‐less first‐order transition at 340(2) K, the structure slightly distorts into the room‐temperature modification with the subgroup Pbca (oP88), and the hydrogen atoms order. The rubidium and caesium compounds are isostructural to each other but not to the potassium compound, and are always obtained as mixtures of two modifications with space groups Cmce (oC88′) and Immb (oI88). Upon heating, the oxohydroxoferrates decompose into their anhydrides AFeO2 and water. The type of hydrogen bonding network influences the decomposition temperature, the structure and the morphology of the crystals. Despite the presence of iron(III), which was confirmed by 57Fe‐Mössbauer spectroscopy, K2[Fe2O3(OH)2] is diamagnetic in the investigated temperature range between 1.8 and 300 K. Neutron diffraction revealed strong antiferromagnetic coupling of the magnetic moments, which are inverted in neighboring tetrahedra.
中文翻译:

具有相关氢键系统的反铁磁性碱金属氧合氢氧铁酸盐(III)。
在水通量条件下合成了氧合氢氧铁酸盐(III)A 2 [Fe 2 O 3(OH)2 ](A = K,Rb,Cs)。大致碱金属氢氧化物和水的等摩尔混合物与使用Fe(NO反应3)3 ⋅9H 2 O不连到约200℃。产物的形成取决于氢氧化物的浓度,因此也取决于其他反应产物,例如KFeO 2,K 2-x [Fe 4 O 7-x(OH)x ]或α ‐Fe 2 O 3。获得。含氧氢氧铁酸盐(III)A 2 [Fe 2 O 3(OH)2 ]的晶体结构遵循相同的结构原理,但根据A和温度的不同,它们的层堆叠或/和氢键系统不同。在得到的四种不同的正交结构类型中,[FeO 3 OH] 4-四面体共享其氧化角,以生成折叠的 Fe 2 O 3(OH)2 ] 2-层。末端氢氧化物配体在层之间和/或之内形成氢键。这些网络中氢原子的位置是相关的。的甲+阳离子位于折叠阴离子层,以及在其沟槽之间。在反应条件下,钾化合物在空间群Cmce(Pearson符号oC 88)中结晶,显示出氢桥中氢原子的双峰无序。在340(2)K处几乎没有磁滞的一阶跃迁中,该结构在Pbca(oP88),氢原子顺序。的铷和铯化合物是同构彼此但不与钾化合物,并且总是与空间群获得的两个修饰混合物CMCE(摄氏度88')和Immb(OI 88)。加热时,氧合氢氧铁酸盐分解成它们的酸酐A FeO 2和水。氢键网络的类型影响分解温度,晶体的结构和形态。尽管存在铁(III),其通过确认57的Fe-穆斯堡尔谱,K 2的[Fe 2 ø 3(OH)2在研究的温度范围为1.8到300 K之间,是抗磁性的。中子衍射揭示了磁矩的强反铁磁耦合,这些磁矩在相邻的四面体中反转。
Fe 2 O 3(OH)2 ] 2-层。末端氢氧化物配体在层之间和/或之内形成氢键。这些网络中氢原子的位置是相关的。的甲+阳离子位于折叠阴离子层,以及在其沟槽之间。在反应条件下,钾化合物在空间群Cmce(Pearson符号oC 88)中结晶,显示出氢桥中氢原子的双峰无序。在340(2)K处几乎没有磁滞的一阶跃迁中,该结构在Pbca(oP88),氢原子顺序。的铷和铯化合物是同构彼此但不与钾化合物,并且总是与空间群获得的两个修饰混合物CMCE(摄氏度88')和Immb(OI 88)。加热时,氧合氢氧铁酸盐分解成它们的酸酐A FeO 2和水。氢键网络的类型影响分解温度,晶体的结构和形态。尽管存在铁(III),其通过确认57的Fe-穆斯堡尔谱,K 2的[Fe 2 ø 3(OH)2在研究的温度范围为1.8到300 K之间,是抗磁性的。中子衍射揭示了磁矩的强反铁磁耦合,这些磁矩在相邻的四面体中反转。
更新日期:2019-11-26
 Fe2O3(OH)2]2− layers. The terminal hydroxide ligands form hydrogen bonds between and/or within the layers. The positions of the hydrogen atoms in these networks are correlated. The A+ cations are located between the folded anionic layers as well as in their trenches. Under reaction conditions, the potassium compound crystallizes in the space group Cmce (Pearson symbol oC88), showing a bimodal disorder of the hydrogen atoms in hydrogen bridges. In a virtually hysteresis‐less first‐order transition at 340(2) K, the structure slightly distorts into the room‐temperature modification with the subgroup Pbca (oP88), and the hydrogen atoms order. The rubidium and caesium compounds are isostructural to each other but not to the potassium compound, and are always obtained as mixtures of two modifications with space groups Cmce (oC88′) and Immb (oI88). Upon heating, the oxohydroxoferrates decompose into their anhydrides AFeO2 and water. The type of hydrogen bonding network influences the decomposition temperature, the structure and the morphology of the crystals. Despite the presence of iron(III), which was confirmed by 57Fe‐Mössbauer spectroscopy, K2[Fe2O3(OH)2] is diamagnetic in the investigated temperature range between 1.8 and 300 K. Neutron diffraction revealed strong antiferromagnetic coupling of the magnetic moments, which are inverted in neighboring tetrahedra.
Fe2O3(OH)2]2− layers. The terminal hydroxide ligands form hydrogen bonds between and/or within the layers. The positions of the hydrogen atoms in these networks are correlated. The A+ cations are located between the folded anionic layers as well as in their trenches. Under reaction conditions, the potassium compound crystallizes in the space group Cmce (Pearson symbol oC88), showing a bimodal disorder of the hydrogen atoms in hydrogen bridges. In a virtually hysteresis‐less first‐order transition at 340(2) K, the structure slightly distorts into the room‐temperature modification with the subgroup Pbca (oP88), and the hydrogen atoms order. The rubidium and caesium compounds are isostructural to each other but not to the potassium compound, and are always obtained as mixtures of two modifications with space groups Cmce (oC88′) and Immb (oI88). Upon heating, the oxohydroxoferrates decompose into their anhydrides AFeO2 and water. The type of hydrogen bonding network influences the decomposition temperature, the structure and the morphology of the crystals. Despite the presence of iron(III), which was confirmed by 57Fe‐Mössbauer spectroscopy, K2[Fe2O3(OH)2] is diamagnetic in the investigated temperature range between 1.8 and 300 K. Neutron diffraction revealed strong antiferromagnetic coupling of the magnetic moments, which are inverted in neighboring tetrahedra.
中文翻译:

具有相关氢键系统的反铁磁性碱金属氧合氢氧铁酸盐(III)。
在水通量条件下合成了氧合氢氧铁酸盐(III)A 2 [Fe 2 O 3(OH)2 ](A = K,Rb,Cs)。大致碱金属氢氧化物和水的等摩尔混合物与使用Fe(NO反应3)3 ⋅9H 2 O不连到约200℃。产物的形成取决于氢氧化物的浓度,因此也取决于其他反应产物,例如KFeO 2,K 2-x [Fe 4 O 7-x(OH)x ]或α ‐Fe 2 O 3。获得。含氧氢氧铁酸盐(III)A 2 [Fe 2 O 3(OH)2 ]的晶体结构遵循相同的结构原理,但根据A和温度的不同,它们的层堆叠或/和氢键系统不同。在得到的四种不同的正交结构类型中,[FeO 3 OH] 4-四面体共享其氧化角,以生成折叠的
 Fe 2 O 3(OH)2 ] 2-层。末端氢氧化物配体在层之间和/或之内形成氢键。这些网络中氢原子的位置是相关的。的甲+阳离子位于折叠阴离子层,以及在其沟槽之间。在反应条件下,钾化合物在空间群Cmce(Pearson符号oC 88)中结晶,显示出氢桥中氢原子的双峰无序。在340(2)K处几乎没有磁滞的一阶跃迁中,该结构在Pbca(oP88),氢原子顺序。的铷和铯化合物是同构彼此但不与钾化合物,并且总是与空间群获得的两个修饰混合物CMCE(摄氏度88')和Immb(OI 88)。加热时,氧合氢氧铁酸盐分解成它们的酸酐A FeO 2和水。氢键网络的类型影响分解温度,晶体的结构和形态。尽管存在铁(III),其通过确认57的Fe-穆斯堡尔谱,K 2的[Fe 2 ø 3(OH)2在研究的温度范围为1.8到300 K之间,是抗磁性的。中子衍射揭示了磁矩的强反铁磁耦合,这些磁矩在相邻的四面体中反转。
Fe 2 O 3(OH)2 ] 2-层。末端氢氧化物配体在层之间和/或之内形成氢键。这些网络中氢原子的位置是相关的。的甲+阳离子位于折叠阴离子层,以及在其沟槽之间。在反应条件下,钾化合物在空间群Cmce(Pearson符号oC 88)中结晶,显示出氢桥中氢原子的双峰无序。在340(2)K处几乎没有磁滞的一阶跃迁中,该结构在Pbca(oP88),氢原子顺序。的铷和铯化合物是同构彼此但不与钾化合物,并且总是与空间群获得的两个修饰混合物CMCE(摄氏度88')和Immb(OI 88)。加热时,氧合氢氧铁酸盐分解成它们的酸酐A FeO 2和水。氢键网络的类型影响分解温度,晶体的结构和形态。尽管存在铁(III),其通过确认57的Fe-穆斯堡尔谱,K 2的[Fe 2 ø 3(OH)2在研究的温度范围为1.8到300 K之间,是抗磁性的。中子衍射揭示了磁矩的强反铁磁耦合,这些磁矩在相邻的四面体中反转。











































 京公网安备 11010802027423号
京公网安备 11010802027423号