当前位置:
X-MOL 学术
›
Macromol. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigation of Bimetallic Nickel Catalysts in Catalyst‐Transfer Polymerization of π‐Conjugated Polymers
Macromolecular Chemistry and Physics ( IF 2.5 ) Pub Date : 2019-11-28 , DOI: 10.1002/macp.201900363 Jeffrey Buenaflor 1 , Parker Sommerville 1 , Hang Qian 2 , Christine Luscombe 2
Macromolecular Chemistry and Physics ( IF 2.5 ) Pub Date : 2019-11-28 , DOI: 10.1002/macp.201900363 Jeffrey Buenaflor 1 , Parker Sommerville 1 , Hang Qian 2 , Christine Luscombe 2
Affiliation
|
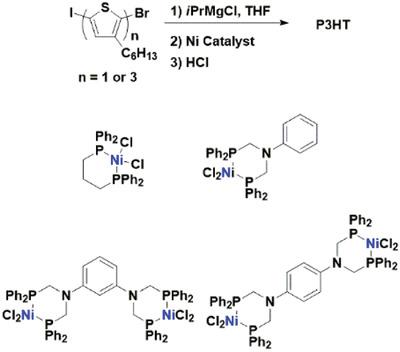
A comparative study involving bimetallic nickel catalysts designed from disubstituted N,N,N′,N′‐tetra(diphenylphosphanylmethyl)benzene diamine bridging ligands is reported. Catalyst behavior is explored in the Kumada catalyst‐transfer polymerization (KCTP) using poly(3‐hexylthiophene) (P3HT) as the model system. The success of a controlled polymerization is monitored by analyzing monomer conversion, degree of polymerization, end‐group identity, and molecular weight distribution. The characterization of P3HT obtained from KCTP initiated with the bimetallic catalysts shows chain‐growth behavior; however, the presence of Br/Br end‐groups and broader molecular weight distribution reveals a reduced controlled polymerization compared to the commonly employed Ni(dppp)Cl2. The observed increase in intermolecular chain transfer and termination processes in KCTP initiation with the bimetallic catalysts can be attributed to a weaker Ni(0)‐π‐aryl complex interaction, which is caused by increased steric crowding of the coordination sphere.
中文翻译:

π共轭聚合物的催化剂转移聚合中双金属镍催化剂的研究
报道了一项涉及由双取代的N,N,N',N'-四(二苯基膦烷基甲基)苯二胺桥联配体设计的双金属镍催化剂的比较研究。使用聚(3-己基噻吩)(P3HT)作为模型系统,在Kumada催化剂转移聚合(KCTP)中探索了催化剂的行为。通过分析单体转化率,聚合度,端基同一性和分子量分布,可以监控受控聚合的成功性。由双金属催化剂引发的从KCTP中获得的P3HT的表征显示了链增长行为。然而,与常用的Ni(dppp)Cl 2相比,Br / Br端基的存在和较宽的分子量分布表明可控聚合降低。。在双金属催化剂的KCTP引发中观察到的分子间链转移和终止过程的增加可归因于Ni(0)-π-芳基络合物的相互作用较弱,这是由于配位球的空间拥挤增加所致。
更新日期:2019-11-28
中文翻译:

π共轭聚合物的催化剂转移聚合中双金属镍催化剂的研究
报道了一项涉及由双取代的N,N,N',N'-四(二苯基膦烷基甲基)苯二胺桥联配体设计的双金属镍催化剂的比较研究。使用聚(3-己基噻吩)(P3HT)作为模型系统,在Kumada催化剂转移聚合(KCTP)中探索了催化剂的行为。通过分析单体转化率,聚合度,端基同一性和分子量分布,可以监控受控聚合的成功性。由双金属催化剂引发的从KCTP中获得的P3HT的表征显示了链增长行为。然而,与常用的Ni(dppp)Cl 2相比,Br / Br端基的存在和较宽的分子量分布表明可控聚合降低。。在双金属催化剂的KCTP引发中观察到的分子间链转移和终止过程的增加可归因于Ni(0)-π-芳基络合物的相互作用较弱,这是由于配位球的空间拥挤增加所致。











































 京公网安备 11010802027423号
京公网安备 11010802027423号