当前位置:
X-MOL 学术
›
Energy Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Energy Analysis of Physical Absorption and Chemical Absorption of CO2 in Ionic Liquids
Energy Technology ( IF 3.8 ) Pub Date : 2019-10-01 , DOI: 10.1002/ente.201900529 Yujiao Xie 1 , Gang Liu 1 , Haiwei Nie 1 , Fangyong Yu 1 , Xiaoxue Xing 1 , Hongyou Cui 1
Energy Technology ( IF 3.8 ) Pub Date : 2019-10-01 , DOI: 10.1002/ente.201900529 Yujiao Xie 1 , Gang Liu 1 , Haiwei Nie 1 , Fangyong Yu 1 , Xiaoxue Xing 1 , Hongyou Cui 1
Affiliation
|
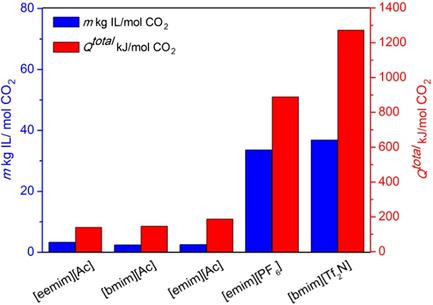
CO2 solubility in ionic liquids (ILs) is measured extensively to develop IL‐based technology for CO2 capture. Herein, the choline‐ and acetate‐based ILs are selected as the studied physical and chemical absorbents for CO2 capture, respectively. The CO2 solubilities in choline‐ and acetate‐based ILs are thermodynamically modeled. The ILs consumed for CO2 absorption and the energy demands for the ILs regeneration are calculated and compared with those of the conventional imidazolium‐based ILs. The results show that the three choline‐based ILs are not energy saving in the pressure‐swing process. In the temperature‐swing process, the energy demand for CO2 absorption by using acetate‐based ILs reveals that the chemical absorption‐based ILs are the absorbent with lower energy demands compared with the physical absorption‐based ILs.
中文翻译:

离子液体中CO2物理吸收和化学吸收的能量分析
广泛测量了CO 2在离子液体(ILs)中的溶解度,以开发基于IL的CO 2捕集技术。在此,分别选择基于胆碱和乙酸盐的ILs作为已研究的物理和化学吸收剂来捕获CO 2。对基于胆碱和乙酸盐的离子液体中的CO 2溶解度进行了热力学建模。计算出吸收CO 2所消耗的IL以及IL再生所需的能量,并将其与传统的咪唑类IL进行比较。结果表明,三个基于胆碱的离子液体在变压过程中均无法节省能源。在温度波动过程中,CO 2的能量需求 通过使用基于乙酸盐的离子液体进行吸收,表明基于化学吸收的离子液体与基于物理吸收的离子液体相比具有较低的能量需求。
更新日期:2019-10-01
中文翻译:

离子液体中CO2物理吸收和化学吸收的能量分析
广泛测量了CO 2在离子液体(ILs)中的溶解度,以开发基于IL的CO 2捕集技术。在此,分别选择基于胆碱和乙酸盐的ILs作为已研究的物理和化学吸收剂来捕获CO 2。对基于胆碱和乙酸盐的离子液体中的CO 2溶解度进行了热力学建模。计算出吸收CO 2所消耗的IL以及IL再生所需的能量,并将其与传统的咪唑类IL进行比较。结果表明,三个基于胆碱的离子液体在变压过程中均无法节省能源。在温度波动过程中,CO 2的能量需求 通过使用基于乙酸盐的离子液体进行吸收,表明基于化学吸收的离子液体与基于物理吸收的离子液体相比具有较低的能量需求。



























 京公网安备 11010802027423号
京公网安备 11010802027423号