当前位置:
X-MOL 学术
›
ChemNanoMat
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Catalytic Oxidation of CO on Sulfur‐Doped Boron Nitride
ChemNanoMat ( IF 2.6 ) Pub Date : 2019-11-27 , DOI: 10.1002/cnma.201900516 Bingping Liu 1 , Jin Yong Lee 2 , Shihai Yan 1
ChemNanoMat ( IF 2.6 ) Pub Date : 2019-11-27 , DOI: 10.1002/cnma.201900516 Bingping Liu 1 , Jin Yong Lee 2 , Shihai Yan 1
Affiliation
|
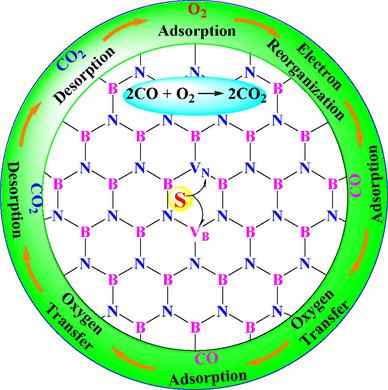
Bridging the gap between homogeneous and heterogeneous catalysis, the single‐atom catalyst supported on a substrate shows extremely high atom efficiency, low cost, excellent stability, and high activity for CO oxidation. Here, we report the catalytic mechanism of CO oxidation on sulfur‐doped h‐BN. The chemisorption activates O2 via electron transfer from sulfur‐doped h‐BN, inducing a reduction in the bond order of O2 and lengthening the O−O distance. This variation is helpful for the subsequent oxidation of CO. The oxidation process includes O2 adsorption, electron reorganization, and two instances each of CO adsorption, oxygen transfer, and CO2 desorption. The first oxygen transfer from O2 to CO occurs exothermically by ∼−63.8 kcal/mol with a barrier of ∼12.1 kcal/mol, which is thus the rate‐determining step. The significantly enhanced catalytic performance implies that sulfur doping on h‐BN should have potential applications in the areas of metal‐free catalysts with low cost and high activity.
中文翻译:

硫掺杂氮化硼对CO的增强催化氧化作用
弥补了均相催化和异相催化之间的空白,负载在基材上的单原子催化剂显示出极高的原子效率,低成本,出色的稳定性和高的CO氧化活性。在这里,我们报告了CO氧化对硫掺杂h-BN的催化机理。化学吸附作用是通过电子从硫掺杂的h-BN转移而激活O 2,从而降低O 2的键序并延长O-O距离。这种变化有助于后续的CO氧化。氧化过程包括O 2吸附,电子重组以及CO吸附,氧转移和CO 2的两种情况解吸。从O 2到CO的首次氧气转移以〜-63.8 kcal / mol的热量放热,其势垒为〜12.1 kcal / mol,因此是速率确定的步骤。催化性能的显着提高表明,在h-BN上掺杂硫应该在低成本和高活性的无金属催化剂领域中具有潜在的应用。
更新日期:2019-11-27
中文翻译:

硫掺杂氮化硼对CO的增强催化氧化作用
弥补了均相催化和异相催化之间的空白,负载在基材上的单原子催化剂显示出极高的原子效率,低成本,出色的稳定性和高的CO氧化活性。在这里,我们报告了CO氧化对硫掺杂h-BN的催化机理。化学吸附作用是通过电子从硫掺杂的h-BN转移而激活O 2,从而降低O 2的键序并延长O-O距离。这种变化有助于后续的CO氧化。氧化过程包括O 2吸附,电子重组以及CO吸附,氧转移和CO 2的两种情况解吸。从O 2到CO的首次氧气转移以〜-63.8 kcal / mol的热量放热,其势垒为〜12.1 kcal / mol,因此是速率确定的步骤。催化性能的显着提高表明,在h-BN上掺杂硫应该在低成本和高活性的无金属催化剂领域中具有潜在的应用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号