当前位置:
X-MOL 学术
›
ChemNanoMat
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoelectrochemical Cells for Artificial Photosynthesis: Alternatives to Water Oxidation
ChemNanoMat ( IF 2.6 ) Pub Date : 2020-01-09 , DOI: 10.1002/cnma.201900616 Andrew Yun Ru Ng 1 , Bhanupriya Boruah 1, 2 , Kek Foo Chin 1 , Jayant M. Modak 2 , Han Sen Soo 1, 3
ChemNanoMat ( IF 2.6 ) Pub Date : 2020-01-09 , DOI: 10.1002/cnma.201900616 Andrew Yun Ru Ng 1 , Bhanupriya Boruah 1, 2 , Kek Foo Chin 1 , Jayant M. Modak 2 , Han Sen Soo 1, 3
Affiliation
|
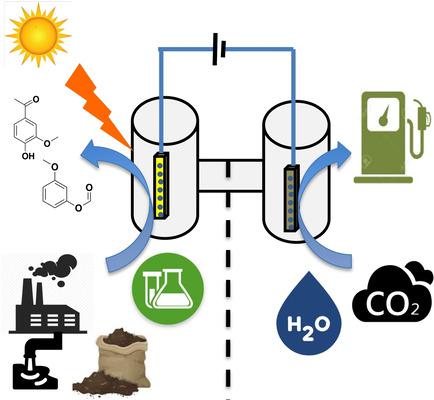
Photoelectrochemical cells have been used as one of the most common artificial photosynthetic approaches to mimic natural photosynthetic water splitting reactions. However, despite the tremendous advances made to improve the affordability and efficiency of photoelectrochemical water splitting, it is still not an economically feasible method to produce solar fuels currently since only the H2 evolving reduction half‐reaction generates valuable fuels. Therefore, in this review, we intend to highlight other underexplored substrates and reactions for producing solar fuels in photoelectrochemical cells, as well as alternative architectures including temporally independent and biohybrid systems. We show that besides water oxidation, electrocatalytic or photoredox reactions for pollutant degradation, biomass valorization, and organic chemical synthesis can be or have been successfully adapted for photoelectrochemical cells, thus offering a virtually infinite number of possibilities for artificial photosynthetic applications which generate valuable products in both the reduction and oxidation half reactions.
中文翻译:

人工光合作用的光电化学电池:水氧化的替代方法
光电化学电池已被用作模拟自然光合水分解反应的最常见的人工光合方法之一。然而,尽管在改善光电化学水分解的可负担性和效率方面取得了巨大的进步,但是由于仅H 2,目前它仍然不是一种经济可行的生产太阳能燃料的方法。不断发展的还原半反应产生了宝贵的燃料。因此,在这篇综述中,我们打算重点介绍其他未开发的底物和在光电化学电池中生产太阳能的反应,以及包括时间独立和生物混合系统在内的替代体系结构。我们表明,除了水氧化以外,用于污染物降解的电催化或光氧化还原反应,生物质平衡和有机化学合成也可以或已经成功地应用于光电化学电池,从而为在人造光合作用中产生有价值的产物提供了几乎无限的可能性。还原和氧化半反应。
更新日期:2020-01-09
中文翻译:

人工光合作用的光电化学电池:水氧化的替代方法
光电化学电池已被用作模拟自然光合水分解反应的最常见的人工光合方法之一。然而,尽管在改善光电化学水分解的可负担性和效率方面取得了巨大的进步,但是由于仅H 2,目前它仍然不是一种经济可行的生产太阳能燃料的方法。不断发展的还原半反应产生了宝贵的燃料。因此,在这篇综述中,我们打算重点介绍其他未开发的底物和在光电化学电池中生产太阳能的反应,以及包括时间独立和生物混合系统在内的替代体系结构。我们表明,除了水氧化以外,用于污染物降解的电催化或光氧化还原反应,生物质平衡和有机化学合成也可以或已经成功地应用于光电化学电池,从而为在人造光合作用中产生有价值的产物提供了几乎无限的可能性。还原和氧化半反应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号