当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective Intermolecular [4+2] Process of N,O‐acetals with Terminal Alkynes for Construction of Functional cis‐Pyrido and Pyrrolo[1,2‐c][1,3]oxazin‐1‐ones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-12-23 , DOI: 10.1002/adsc.201901141 Chen Wang 1 , Zhuo‐Ya Mao 1 , Yi‐Wen Liu 1 , Qiao‐E Wang 2 , Chang‐Mei Si 1 , Bang‐Guo Wei 1 , Guo‐Qiang Lin 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-12-23 , DOI: 10.1002/adsc.201901141 Chen Wang 1 , Zhuo‐Ya Mao 1 , Yi‐Wen Liu 1 , Qiao‐E Wang 2 , Chang‐Mei Si 1 , Bang‐Guo Wei 1 , Guo‐Qiang Lin 3
Affiliation
|
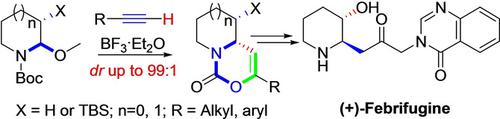
A diastereoselective approach to access cis‐pyrido and pyrrolo[1,2‐c][1,3]oxazin‐1‐ones has been developed through a one‐pot BF3.Et2O‐catalyzed [4+2] process starting with N,O‐acetals and terminal alkynes. In addition, the utility of this transformation is demonstrated by the scalable synthesis of (+)‐Febrifugine in 7 steps from the N,O‐acetal (15% overall yield).
中文翻译:

N,O-乙缩醛与末端炔烃的立体选择性分子间[4 + 2]过程,用于构建功能性顺-吡咯基和吡咯并[1,2-c] [1,3]恶嗪-1-酮
通过单锅BF 3开发了一种非对映选择性的方法来获得顺式吡啶基和吡咯并[1,2-c] [1,3]恶嗪-1-酮。从N,O-乙缩醛和末端炔烃开始的Et 2 O催化的[4 + 2]过程。此外,通过从N,O-乙缩醛分7步(总收率15%)的可扩展合成(+)-Febrifugine证明了这种转化的效用。
更新日期:2019-12-23
中文翻译:

N,O-乙缩醛与末端炔烃的立体选择性分子间[4 + 2]过程,用于构建功能性顺-吡咯基和吡咯并[1,2-c] [1,3]恶嗪-1-酮
通过单锅BF 3开发了一种非对映选择性的方法来获得顺式吡啶基和吡咯并[1,2-c] [1,3]恶嗪-1-酮。从N,O-乙缩醛和末端炔烃开始的Et 2 O催化的[4 + 2]过程。此外,通过从N,O-乙缩醛分7步(总收率15%)的可扩展合成(+)-Febrifugine证明了这种转化的效用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号