当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
C1 Oxidation/C2 Reduction Isomerization of Unprotected Aldoses Induced by Light/Ketone.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-10 , DOI: 10.1002/anie.201914242 Yusuke Masuda 1 , Hiromu Tsuda 1 , Masahiro Murakami 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-12-10 , DOI: 10.1002/anie.201914242 Yusuke Masuda 1 , Hiromu Tsuda 1 , Masahiro Murakami 1
Affiliation
|
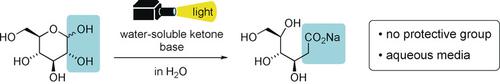
Unprotected aldoses in water undergo an isomerization reaction via a radical pathway when irradiated with light in the presence of water-soluble benzophenone. Whereas its anomeric carbon (C1) is oxidized to a carboxy group, the hydroxy group on the C2 carbon is replaced by hydrogen. The generated 2-deoxy lactones are readily reduced to the corresponding 2-deoxy aldoses, which are often contained in bioactive compounds.
中文翻译:

光/酮诱导的未保护的醛糖的C1氧化/ C2还原异构化。
当在水溶性二苯甲酮存在下用光照射时,水中未保护的醛糖会通过自由基途径经历异构化反应。而其异头碳(C1)被氧化成羧基,而C2碳上的羟基被氢取代。产生的2-脱氧内酯很容易还原为相应的2-脱氧醛糖,通常包含在生物活性化合物中。
更新日期:2020-01-16
中文翻译:

光/酮诱导的未保护的醛糖的C1氧化/ C2还原异构化。
当在水溶性二苯甲酮存在下用光照射时,水中未保护的醛糖会通过自由基途径经历异构化反应。而其异头碳(C1)被氧化成羧基,而C2碳上的羟基被氢取代。产生的2-脱氧内酯很容易还原为相应的2-脱氧醛糖,通常包含在生物活性化合物中。









































 京公网安备 11010802027423号
京公网安备 11010802027423号