当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
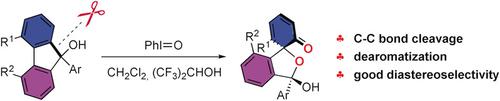
Hypervalent-Iodine-Mediated Carbon-Carbon Bond Cleavage and Dearomatization of 9H-Fluoren-9-ols.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2019-12-10 , DOI: 10.1002/anie.201913373 Ruixian Deng 1 , Shuming Zhan 1 , Chunyu Li 1 , Zhenhua Gu 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2019-12-10 , DOI: 10.1002/anie.201913373 Ruixian Deng 1 , Shuming Zhan 1 , Chunyu Li 1 , Zhenhua Gu 1
Affiliation
|
A transition-metal-free synthesis of spiro compounds from 9H-fluoren-9-ols mediated by hypervalent iodine is reported. In this reaction, an unprecedented β-carbon elimination of tertiary alkoxyliodine(III) to form new diaryliodonium salts is proposed. The obtained phenol intermediates undergo oxidative dearomatization to furnish a class of oxo-spiro compounds. This domino reaction significantly increases the complexity of these molecules and shows excellent regio- and stereoselectivity.
中文翻译:

高价碘介导的碳-碳键裂解和9H-Fluoren-9-ols脱芳香化。
据报道,由高价碘介导的9H-芴-9-ols可以无过渡金属合成螺环化合物。在该反应中,提出了前所未有的β-碳消除叔烷氧基碘(III)以形成新的二芳基碘鎓盐的方法。所获得的苯酚中间体经过氧化脱芳香化作用以提供一类氧代-螺环化合物。这种多米诺骨牌反应显着增加了这些分子的复杂性,并显示出极好的区域选择性和立体选择性。
更新日期:2020-01-16
中文翻译:

高价碘介导的碳-碳键裂解和9H-Fluoren-9-ols脱芳香化。
据报道,由高价碘介导的9H-芴-9-ols可以无过渡金属合成螺环化合物。在该反应中,提出了前所未有的β-碳消除叔烷氧基碘(III)以形成新的二芳基碘鎓盐的方法。所获得的苯酚中间体经过氧化脱芳香化作用以提供一类氧代-螺环化合物。这种多米诺骨牌反应显着增加了这些分子的复杂性,并显示出极好的区域选择性和立体选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号