当前位置:
X-MOL 学术
›
Cell Host Microbe
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis for a Species-Specific Determinant of an SIV Vif Protein toward Hominid APOBEC3G Antagonism.
Cell Host & Microbe ( IF 20.6 ) Pub Date : 2019-12-11 , DOI: 10.1016/j.chom.2019.10.014 Jennifer M Binning 1 , Nicholas M Chesarino 2 , Michael Emerman 2 , John D Gross 1
Cell Host & Microbe ( IF 20.6 ) Pub Date : 2019-12-11 , DOI: 10.1016/j.chom.2019.10.014 Jennifer M Binning 1 , Nicholas M Chesarino 2 , Michael Emerman 2 , John D Gross 1
Affiliation
|
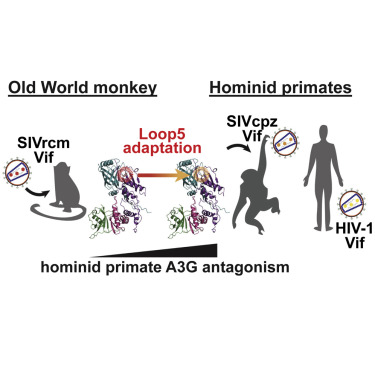
Primate lentiviruses encode a Vif protein that counteracts the host antiviral APOBEC3 (A3) family members. The adaptation of Vif to species-specific A3 determinants is a critical event that allowed the spillover of a lentivirus from monkey reservoirs to chimpanzees and subsequently to humans, which gave rise to HIV-1 and the acquired immune deficiency syndrome (AIDS) pandemic. How Vif-A3 protein interactions are remodeled during evolution is unclear. Here, we report a 2.94 Å crystal structure of the Vif substrate receptor complex from simian immunodeficiency virus isolated from red-capped mangabey (SIVrcm). The structure of the SIVrcm Vif complex illuminates the stage of lentiviral Vif evolution that is immediately prior to entering hominid primates. Structure-function studies reveal the adaptations that allowed SIVrcm Vif to antagonize hominid A3G. These studies show a partitioning between an evolutionarily dynamic specificity determinant and a conserved protein interacting surface on Vif that enables adaptation while maintaining protein interactions required for potent A3 antagonism.
中文翻译:

SIV Vif蛋白针对人源APOBEC3G拮抗作用的物种特异性决定簇的结构基础。
灵长类慢病毒编码与宿主抗病毒APOBEC3(A3)家族成员抵消的Vif蛋白。Vif对物种特异性A3决定簇的适应性变化至关重要,这使慢病毒从猴子水库溢出到黑猩猩,然后又扩散到人类,这导致HIV-1和后天免疫机能丧失综合症(AIDS)大流行。尚不清楚进化过程中如何重塑Vif-A3蛋白相互作用。在这里,我们报道了猿猴免疫缺陷病毒Vif底物受体复合物的2.94Å晶体结构,该病毒是从红顶芒果(SIVrcm)中分离出来的。SIVrcm Vif复合体的结构阐明了慢病毒Vif进化的阶段,该阶段就在进入原始人灵长类动物之前。结构功能研究揭示了使SIVrcm Vif拮抗人源A3G的适应性。这些研究表明,在进化动力学特异性决定因子和Vif上保守的蛋白质相互作用表面之间存在分隔,从而可以进行适应,同时保持有效的A3拮抗作用所需的蛋白质相互作用。
更新日期:2019-12-11
中文翻译:

SIV Vif蛋白针对人源APOBEC3G拮抗作用的物种特异性决定簇的结构基础。
灵长类慢病毒编码与宿主抗病毒APOBEC3(A3)家族成员抵消的Vif蛋白。Vif对物种特异性A3决定簇的适应性变化至关重要,这使慢病毒从猴子水库溢出到黑猩猩,然后又扩散到人类,这导致HIV-1和后天免疫机能丧失综合症(AIDS)大流行。尚不清楚进化过程中如何重塑Vif-A3蛋白相互作用。在这里,我们报道了猿猴免疫缺陷病毒Vif底物受体复合物的2.94Å晶体结构,该病毒是从红顶芒果(SIVrcm)中分离出来的。SIVrcm Vif复合体的结构阐明了慢病毒Vif进化的阶段,该阶段就在进入原始人灵长类动物之前。结构功能研究揭示了使SIVrcm Vif拮抗人源A3G的适应性。这些研究表明,在进化动力学特异性决定因子和Vif上保守的蛋白质相互作用表面之间存在分隔,从而可以进行适应,同时保持有效的A3拮抗作用所需的蛋白质相互作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号