当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereochemistry in the Reaction of the myo-Inositol Phosphate Synthase Ortholog Ari2 during Aristeromycin Biosynthesis.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-13 , DOI: 10.1021/acs.biochem.9b00981 Fumitaka Kudo 1 , Takeshi Tsunoda 1 , Kaito Yamaguchi 1 , Akimasa Miyanaga 1 , Tadashi Eguchi 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-13 , DOI: 10.1021/acs.biochem.9b00981 Fumitaka Kudo 1 , Takeshi Tsunoda 1 , Kaito Yamaguchi 1 , Akimasa Miyanaga 1 , Tadashi Eguchi 1
Affiliation
|
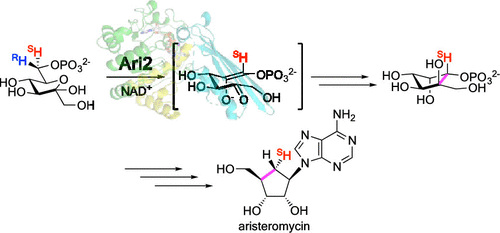
The myo-inositol-1-phosphate synthase (MIPS) ortholog Ari2, which is encoded in the aristeromycin biosynthetic gene cluster, catalyzes the formation of five-membered cyclitol phosphate using d-fructose 6-phosphate (F6P) as a substrate. To understand the stereochemistry during the Ari2 reaction in vivo, we carried out feeding experiments with (6S)-d-[6-2H1]- and (6R)-d-[6-2H1]glucose in the aristeromycin-producing strain Streptomyces citricolor. We observed retention of the 2H atom of (6S)-d-[6-2H1]glucose and no incorporation of the 2H atom from (6R)-d-[6-2H1]glucose in aristeromycin. This indicates that Ari2 abstracts the pro-R proton at C6 of F6P after oxidation of C5-OH by nicotinamide adenine dinucleotide (NAD+) to generate the enolate intermediate, which then attacks the C2 ketone to form the C-C bond via aldol-type condensation. The reaction of Ari2 with (6S)-d-[6-2H1]- and (6R)-d-[6-2H1]F6P in vitro exhibited identical stereochemistry compared with that observed during the feeding experiments. Furthermore, analysis of the crystal structure of Ari2, including NAD+ as a ligand, revealed the active site of Ari2 to be similar to that of MIPS of Mycobacterium tuberculosis, supporting the similarity of the reaction mechanisms of Ari2 and MIPS.
中文翻译:

阿奇霉素生物合成过程中肌-肌醇磷酸合酶Ortholog Ari2反应的立体化学。
在阿霉素生物合成基因簇中编码的肌醇-1-磷酸合酶(MIPS)直向同源物Ari2以d-果糖6-磷酸酯(F6P)为底物催化五元环磷酸磷酸酯的形成。为了解体内Ari2反应过程中的立体化学,我们在产生阿霉素的菌株柠檬色链霉菌中用(6S)-d- [6-2H1]-和(6R)-d- [6-2H1]葡萄糖进行了饲养实验。我们观察到在阿霉素中保留了(6S)-d- [6-2H1]葡萄糖的2H原子,并且没有引入来自(6R)-d- [6-2H1]葡萄糖的2H原子。这表明在通过烟酰胺腺嘌呤二核苷酸(NAD +)氧化C5-OH生成烯醇化物中间体后,Ari2在F6P的C6处提取质子R质子,生成烯醇盐中间体,然后该中间体通过羟醛缩合攻击C2酮形成CC键。与饲喂实验中观察到的相比,Ari2与(6S)-d- [6-2H1]-和(6R)-d- [6-2H1] F6P的体外反应显示出相同的立体化学。此外,对Ari2的晶体结构(包括NAD +作为配体)的分析显示,Ari2的活性位点与结核分枝杆菌的MIPS的活性位点相似,从而支持了Ari2和MIPS的反应机理的相似性。
更新日期:2019-12-17
中文翻译:

阿奇霉素生物合成过程中肌-肌醇磷酸合酶Ortholog Ari2反应的立体化学。
在阿霉素生物合成基因簇中编码的肌醇-1-磷酸合酶(MIPS)直向同源物Ari2以d-果糖6-磷酸酯(F6P)为底物催化五元环磷酸磷酸酯的形成。为了解体内Ari2反应过程中的立体化学,我们在产生阿霉素的菌株柠檬色链霉菌中用(6S)-d- [6-2H1]-和(6R)-d- [6-2H1]葡萄糖进行了饲养实验。我们观察到在阿霉素中保留了(6S)-d- [6-2H1]葡萄糖的2H原子,并且没有引入来自(6R)-d- [6-2H1]葡萄糖的2H原子。这表明在通过烟酰胺腺嘌呤二核苷酸(NAD +)氧化C5-OH生成烯醇化物中间体后,Ari2在F6P的C6处提取质子R质子,生成烯醇盐中间体,然后该中间体通过羟醛缩合攻击C2酮形成CC键。与饲喂实验中观察到的相比,Ari2与(6S)-d- [6-2H1]-和(6R)-d- [6-2H1] F6P的体外反应显示出相同的立体化学。此外,对Ari2的晶体结构(包括NAD +作为配体)的分析显示,Ari2的活性位点与结核分枝杆菌的MIPS的活性位点相似,从而支持了Ari2和MIPS的反应机理的相似性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号