当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Linear Well-Defined Polyamines via Anionic Ring-Opening Polymerization of Activated Aziridines: From Mild Desulfonylation to Cell Transfection
ACS Macro Letters ( IF 5.1 ) Pub Date : 2019-12-11 , DOI: 10.1021/acsmacrolett.9b00792 Tassilo Gleede 1 , Fangzhou Yu 2 , Ying-Li Luo 2 , Youyong Yuan 2 , Jun Wang 2 , Frederik R Wurm 1
ACS Macro Letters ( IF 5.1 ) Pub Date : 2019-12-11 , DOI: 10.1021/acsmacrolett.9b00792 Tassilo Gleede 1 , Fangzhou Yu 2 , Ying-Li Luo 2 , Youyong Yuan 2 , Jun Wang 2 , Frederik R Wurm 1
Affiliation
|
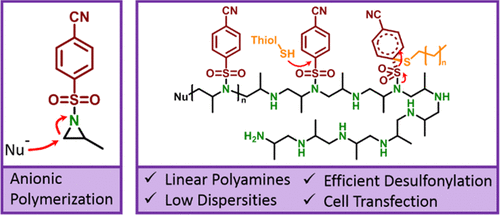
Linear polyethylenimine (L-PEI), a standard for nonviral gene delivery, is usually prepared by hydrolysis from poly(2-oxazoline)s. Lately, anionic polymerization of sulfonamide-activated aziridines had been reported as an alternative pathway toward well-defined L-PEI and linear polyamines. However, desulfonylation of the poly(sulfonyl aziridine)s typically relied on harsh conditions (acid, microwave) or used a toxic solvent (e.g., hexamethylphosphoramide). In addition, the drastic change of polarity requires solvents, which keep poly(sulfonyl aziridine)s as well as L-PEI in solution, and only a limited number of strategies were reported. Herein, we prepared 1-(4-cyanobenzenesulfonyl) 2-methyl-aziridine (1) as a monomer for the anionic ring-opening polymerization. It was polymerized to well-defined and linear poly(sulfonyl aziridine)s. The 4-cyanobenzenesulfonyl-activating groups were removed under mild conditions to linear polypropylenimine (L-PPI). Using dodecanethiol and diazabicyclo-undecene (DBU) allowed ≥98% desulfonylation and a reliable purification toward polyamines with high purity and avoiding main-chain scission. This method represents a fast approach in comparison to previous methods used for postpolymerization desulfonylation and produces linear well-defined polyamines. The high control over molecular weight and dispersities achieved by living anionic polymerization are the key advantages of our strategy, especially if used for biomedical applications, in which molecular weight might correlate with toxicity. The synthesized polypropylenimine was further tested as a cell-transfection agent and proved, with 16.1% transfection efficiency of the cationic nanoparticles, to be an alternative to L-PEI obtained from the 2-oxazoline route. This general strategy will allow the preparation of complex macromolecular architectures containing polyamine segments, which were not accessible before.
中文翻译:

通过活化氮丙啶的阴离子开环聚合获得线性定义明确的多胺:从轻度去磺酰化到细胞转染
线性聚乙烯亚胺 (L-PEI) 是一种非病毒基因传递的标准品,通常由聚 (2-恶唑啉) 水解制备。最近,磺胺活化氮丙啶的阴离子聚合已被报道为获得明确定义的 L-PEI 和线性多胺的替代途径。然而,聚(磺酰基氮丙啶)的脱磺酰化通常依赖于苛刻的条件(酸、微波)或使用有毒溶剂(例如,六甲基磷酰胺)。此外,极性的剧烈变化需要溶剂来保持聚(磺酰基氮丙啶)和 L-PEI 在溶液中,并且只报道了有限数量的策略。在此,我们制备了 1-(4-氰基苯磺酰基) 2-甲基-氮丙啶 ( 1) 作为阴离子开环聚合的单体。它被聚合成定义明确的线性聚(磺酰基氮丙啶)。在温和条件下将 4-氰基苯磺酰基活化基团去除为线性聚丙烯亚胺 (L-PPI)。使用十二硫醇和二氮杂双环十一碳烯 (DBU) 可以实现 ≥98% 的脱磺酰化,并可靠地纯化高纯度的多胺,避免主链断裂。与以前用于后聚合脱磺酰化的方法相比,该方法代表了一种快速方法,并产生了线性明确的多胺。通过活性阴离子聚合实现对分子量和分散性的高度控制是我们策略的关键优势,特别是如果用于生物医学应用,其中分子量可能与毒性相关。合成的聚丙烯亚胺作为细胞转染剂进行了进一步测试,证明阳离子纳米粒子的转染效率为 16.1%,可替代从 2-恶唑啉途径获得的 L-PEI。这种通用策略将允许制备包含多胺链段的复杂大分子结构,这是以前无法获得的。
更新日期:2019-12-11
中文翻译:

通过活化氮丙啶的阴离子开环聚合获得线性定义明确的多胺:从轻度去磺酰化到细胞转染
线性聚乙烯亚胺 (L-PEI) 是一种非病毒基因传递的标准品,通常由聚 (2-恶唑啉) 水解制备。最近,磺胺活化氮丙啶的阴离子聚合已被报道为获得明确定义的 L-PEI 和线性多胺的替代途径。然而,聚(磺酰基氮丙啶)的脱磺酰化通常依赖于苛刻的条件(酸、微波)或使用有毒溶剂(例如,六甲基磷酰胺)。此外,极性的剧烈变化需要溶剂来保持聚(磺酰基氮丙啶)和 L-PEI 在溶液中,并且只报道了有限数量的策略。在此,我们制备了 1-(4-氰基苯磺酰基) 2-甲基-氮丙啶 ( 1) 作为阴离子开环聚合的单体。它被聚合成定义明确的线性聚(磺酰基氮丙啶)。在温和条件下将 4-氰基苯磺酰基活化基团去除为线性聚丙烯亚胺 (L-PPI)。使用十二硫醇和二氮杂双环十一碳烯 (DBU) 可以实现 ≥98% 的脱磺酰化,并可靠地纯化高纯度的多胺,避免主链断裂。与以前用于后聚合脱磺酰化的方法相比,该方法代表了一种快速方法,并产生了线性明确的多胺。通过活性阴离子聚合实现对分子量和分散性的高度控制是我们策略的关键优势,特别是如果用于生物医学应用,其中分子量可能与毒性相关。合成的聚丙烯亚胺作为细胞转染剂进行了进一步测试,证明阳离子纳米粒子的转染效率为 16.1%,可替代从 2-恶唑啉途径获得的 L-PEI。这种通用策略将允许制备包含多胺链段的复杂大分子结构,这是以前无法获得的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号