当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Establishing Cation and Radical Donor Ability Scales of Electrophilic F, CF3, and SCF3 Transfer Reagents.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-12-11 , DOI: 10.1021/acs.accounts.9b00393 Man Li 1 , Xiao-Song Xue 1 , Jin-Pei Cheng 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2019-12-11 , DOI: 10.1021/acs.accounts.9b00393 Man Li 1 , Xiao-Song Xue 1 , Jin-Pei Cheng 1, 2
Affiliation
|
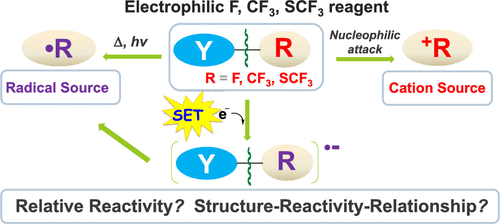
Because of their unique biological, physical, and chemical properties, organofluorine compounds play an increasingly important role in numerous areas of chemistry and everyday life. However, although fluorine is the most abundant halogen in the earth's crust and ranks 13th in abundance among all elements, naturally occurring organofluorine compounds are rare. Consequently, there is a growing demand for the development of safe and efficient reagents and selective synthetic methodologies for the introduction of fluorine or fluorine-containing groups into organic compounds. A wide variety of shelf-stable electrophilic fluorinating and fluoroalkylating reagents have been developed in the past decades. Some of them have also been shown to act as radical sources. These versatile reagents have promoted revolutionary advances in synthetic fluorine chemistry. These developments of novel reagents and the choice of suitable reagents for new reactions have relied largely on the traditional trial-and-error approach because (i) structure-reactivity relationships and mechanisms of reactions of these reagents are sparse and (ii) the rules that govern the synthesis of non-fluorinated analogues cannot necessarily be transposed to fluorinated compounds ( Cahard , D. ; et al. Chem. Soc. Rev. 2014 , 43 , 135 ), since organic fluorine compounds often exhibit unusual properties. Over the past several years, our studies have aimed at establishing comprehensive cation and radical donor scales of electrophilic F, CF3, and SCF3 transfer reagents. We have also developed detailed structure-reactivity relationships. We used density functional theory calculations to systematically investigate the energies required to heterolytically cleave the Y-F/CF3/SCF3 bonds to donate electrophilic F/CF3/SCF3 groups. We found that these energies can be used as convenient indicators of the relative electrophilic fluorinating/trifluoromethylating/trifluoromethylthiolating strengths of these reagents. We have constructed the first comprehensive cation donor scales for electrophilic F, CF3, and SCF3 transfer reagents. In collaboration with Mayr group, we experimentally determined the electrophilicity parameters of SCF3 transfer reagents and demonstrated the importance of intrinsic barriers for predicting their kinetic reactivity. The recognition of the novel application of a few traditional electrophilic reagents as radical sources prompted us further to construct comprehensive radical donor scales of electrophilic F, CF3, and SCF3 transfer reagents. We identified a series of potential new radical F, CF3, and SCF3 donors. Single electron transfer was found to exhibit a substantial effect on activation of the Y-CF3/SCF3 bonds, significantly facilitating the release of CF3/SCF3 radicals. This Account summarizes computational and experimental accomplishments from our group and others to establish the missing links between structure and reactivity for these reagents. Our results pave the way toward the rational optimization, design, and prediction of novel electrophilic fluorinating and fluoroalkylating reagents and new reactions.
中文翻译:

建立亲电F,CF3和SCF3转移试剂的阳离子和自由基供体能力量表。
由于其独特的生物学,物理和化学特性,有机氟化合物在许多化学和日常生活领域中发挥着越来越重要的作用。但是,尽管氟是地壳中最丰富的卤素,并且在所有元素中的丰度都排在第13位,但天然存在的有机氟化合物却很少。因此,对于将氟或含氟基团引入有机化合物中的安全有效的试剂和选择性合成方法的开发需求不断增长。在过去的几十年中,已经开发了各种各样的耐贮存的亲电氟化试剂和氟烷基化试剂。他们中的一些人也被证明是激进的来源。这些通用的试剂促进了合成氟化学的革命性进展。这些新试剂的开发以及为新反应选择合适的试剂很大程度上依赖于传统的试错法,因为(i)这些试剂的结构-反应关系和反应机理稀疏,并且(ii)由于有机氟化合物通常表现出不同寻常的特性,因此,非氟类似物的合成未必一定会转位为氟化化合物(Cahard,D .;等人,Chem.Soc.Rev.2014,43,135)。在过去的几年中,我们的研究旨在建立全面的亲电F,CF3和SCF3转移试剂的阳离子和自由基供体规模。我们还开发了详细的结构-反应关系。我们使用密度泛函理论计算系统地研究了将YF / CF3 / SCF3键杂化裂解以提供亲电F / CF3 / SCF3基团所需的能量。我们发现这些能量可用作这些试剂的相对亲电性氟化/三氟甲基化/三氟甲基硫醇化强度的方便指示剂。我们为亲电F,CF3和SCF3转移试剂构建了第一个全面的阳离子供体秤。我们与Mayr组合作,通过实验确定了SCF3转移试剂的亲电参数,并证明了内在屏障对预测其动力学反应性的重要性。对几种传统亲电试剂作为自由基来源的新颖应用的认识促使我们进一步构建了亲电F,CF3和SCF3转移试剂的综合自由基供体规模。我们确定了一系列潜在的新的自由基F,CF3和SCF3供体。发现单电子转移对Y-CF3 / SCF3键的活化具有实质性影响,从而显着促进了CF3 / SCF3自由基的释放。该帐户总结了我们小组和其他人员的计算和实验成就,以建立这些试剂的结构与反应性之间缺少的联系。我们的结果为新型亲电氟化和氟代烷基化试剂以及新反应的合理优化,设计和预测铺平了道路。和SCF3转移试剂。我们确定了一系列潜在的新的自由基F,CF3和SCF3供体。发现单电子转移对Y-CF3 / SCF3键的活化具有实质性影响,从而显着促进了CF3 / SCF3自由基的释放。该帐户总结了我们小组和其他人员的计算和实验成就,以建立这些试剂的结构与反应性之间缺少的联系。我们的结果为新型亲电氟化和氟代烷基化试剂以及新反应的合理优化,设计和预测铺平了道路。和SCF3转移试剂。我们确定了一系列潜在的新的自由基F,CF3和SCF3供体。发现单电子转移对Y-CF3 / SCF3键的活化具有实质性影响,从而显着促进了CF3 / SCF3自由基的释放。该帐户总结了我们小组和其他人员的计算和实验成就,以建立这些试剂的结构与反应性之间缺少的联系。我们的研究结果为合理优化,设计和预测新型亲电氟化和氟代烷基化试剂以及新反应铺平了道路。大大促进了CF3 / SCF3自由基的释放。该帐户总结了我们小组和其他人员的计算和实验成就,以建立这些试剂的结构与反应性之间缺少的联系。我们的结果为新型亲电氟化和氟代烷基化试剂以及新反应的合理优化,设计和预测铺平了道路。大大促进了CF3 / SCF3自由基的释放。该帐户总结了我们小组和其他人员的计算和实验成就,以建立这些试剂的结构与反应性之间缺少的联系。我们的结果为新型亲电氟化和氟代烷基化试剂以及新反应的合理优化,设计和预测铺平了道路。
更新日期:2019-12-11
中文翻译:

建立亲电F,CF3和SCF3转移试剂的阳离子和自由基供体能力量表。
由于其独特的生物学,物理和化学特性,有机氟化合物在许多化学和日常生活领域中发挥着越来越重要的作用。但是,尽管氟是地壳中最丰富的卤素,并且在所有元素中的丰度都排在第13位,但天然存在的有机氟化合物却很少。因此,对于将氟或含氟基团引入有机化合物中的安全有效的试剂和选择性合成方法的开发需求不断增长。在过去的几十年中,已经开发了各种各样的耐贮存的亲电氟化试剂和氟烷基化试剂。他们中的一些人也被证明是激进的来源。这些通用的试剂促进了合成氟化学的革命性进展。这些新试剂的开发以及为新反应选择合适的试剂很大程度上依赖于传统的试错法,因为(i)这些试剂的结构-反应关系和反应机理稀疏,并且(ii)由于有机氟化合物通常表现出不同寻常的特性,因此,非氟类似物的合成未必一定会转位为氟化化合物(Cahard,D .;等人,Chem.Soc.Rev.2014,43,135)。在过去的几年中,我们的研究旨在建立全面的亲电F,CF3和SCF3转移试剂的阳离子和自由基供体规模。我们还开发了详细的结构-反应关系。我们使用密度泛函理论计算系统地研究了将YF / CF3 / SCF3键杂化裂解以提供亲电F / CF3 / SCF3基团所需的能量。我们发现这些能量可用作这些试剂的相对亲电性氟化/三氟甲基化/三氟甲基硫醇化强度的方便指示剂。我们为亲电F,CF3和SCF3转移试剂构建了第一个全面的阳离子供体秤。我们与Mayr组合作,通过实验确定了SCF3转移试剂的亲电参数,并证明了内在屏障对预测其动力学反应性的重要性。对几种传统亲电试剂作为自由基来源的新颖应用的认识促使我们进一步构建了亲电F,CF3和SCF3转移试剂的综合自由基供体规模。我们确定了一系列潜在的新的自由基F,CF3和SCF3供体。发现单电子转移对Y-CF3 / SCF3键的活化具有实质性影响,从而显着促进了CF3 / SCF3自由基的释放。该帐户总结了我们小组和其他人员的计算和实验成就,以建立这些试剂的结构与反应性之间缺少的联系。我们的结果为新型亲电氟化和氟代烷基化试剂以及新反应的合理优化,设计和预测铺平了道路。和SCF3转移试剂。我们确定了一系列潜在的新的自由基F,CF3和SCF3供体。发现单电子转移对Y-CF3 / SCF3键的活化具有实质性影响,从而显着促进了CF3 / SCF3自由基的释放。该帐户总结了我们小组和其他人员的计算和实验成就,以建立这些试剂的结构与反应性之间缺少的联系。我们的结果为新型亲电氟化和氟代烷基化试剂以及新反应的合理优化,设计和预测铺平了道路。和SCF3转移试剂。我们确定了一系列潜在的新的自由基F,CF3和SCF3供体。发现单电子转移对Y-CF3 / SCF3键的活化具有实质性影响,从而显着促进了CF3 / SCF3自由基的释放。该帐户总结了我们小组和其他人员的计算和实验成就,以建立这些试剂的结构与反应性之间缺少的联系。我们的研究结果为合理优化,设计和预测新型亲电氟化和氟代烷基化试剂以及新反应铺平了道路。大大促进了CF3 / SCF3自由基的释放。该帐户总结了我们小组和其他人员的计算和实验成就,以建立这些试剂的结构与反应性之间缺少的联系。我们的结果为新型亲电氟化和氟代烷基化试剂以及新反应的合理优化,设计和预测铺平了道路。大大促进了CF3 / SCF3自由基的释放。该帐户总结了我们小组和其他人员的计算和实验成就,以建立这些试剂的结构与反应性之间缺少的联系。我们的结果为新型亲电氟化和氟代烷基化试剂以及新反应的合理优化,设计和预测铺平了道路。









































 京公网安备 11010802027423号
京公网安备 11010802027423号