当前位置:
X-MOL 学术
›
JAMA Psychiatry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of Mirtazapine for Methamphetamine Use Disorder Among Cisgender Men and Transgender Women Who Have Sex With Men: A Placebo-Controlled Randomized Clinical Trial.
JAMA Psychiatry ( IF 22.5 ) Pub Date : 2019-12-11 , DOI: 10.1001/jamapsychiatry.2019.3655 Phillip O Coffin 1, 2 , Glenn-Milo Santos 1, 3 , Jaclyn Hern 1 , Eric Vittinghoff 4 , John E Walker 1 , Tim Matheson 1 , Deirdre Santos 1 , Grant Colfax 1 , Steven L Batki 5
JAMA Psychiatry ( IF 22.5 ) Pub Date : 2019-12-11 , DOI: 10.1001/jamapsychiatry.2019.3655 Phillip O Coffin 1, 2 , Glenn-Milo Santos 1, 3 , Jaclyn Hern 1 , Eric Vittinghoff 4 , John E Walker 1 , Tim Matheson 1 , Deirdre Santos 1 , Grant Colfax 1 , Steven L Batki 5
Affiliation
|
Importance
Methamphetamine use is increasingly prevalent and associated with HIV transmission. A previous phase 2a study of mirtazapine demonstrated reductions in methamphetamine use and sexual risk behaviors among men who have sex with men.
Objective
To determine the efficacy of mirtazapine for treatment of methamphetamine use disorder and reduction in HIV risk behaviors.
Design, Setting, and Participants
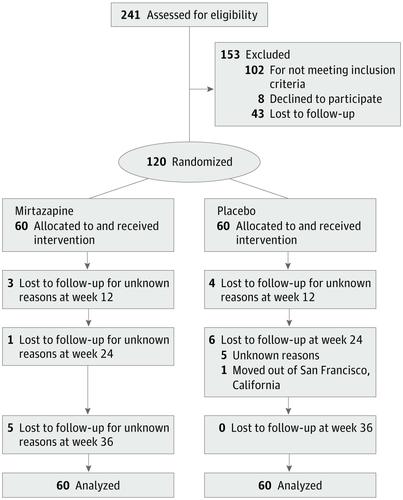
This double-blind randomized clinical trial of mirtazapine vs placebo took place from August 2013 to September 2017 in an outpatient research clinic in San Francisco, California. Participants were community-recruited adults who were sexually active; cisgender men, transgender men, and transgender women who (1) had sex with men, (2) had methamphetamine use disorder, and (3) were actively using methamphetamine were eligible. Participants were randomized to receive the study drug or placebo for 24 weeks, with 12 more weeks of follow-up. Data analysis took place from February to June 2018.
Exposures
Mirtazapine, 30 mg, or matched placebo orally once daily for 24 weeks, with background counseling.
Main Outcomes and Measures
Positive urine test results for methamphetamine over 12, 24, and 36 weeks (primary outcomes) and sexual risk behaviors (secondary outcomes). Sleep, methamphetamine craving, dependence severity, and adverse events were assessed.
Results
Of 241 persons assessed, 120 were enrolled (5 transgender women and 115 cisgender men). The mean (SD) age was 43.3 (9.8) years; 61 (50.8%) were white, 31 (25.8%) were African American, and 15 (12.5%) were Latinx. A mean (SD) of 66% (47%) of visits were completed overall. By week 12, the rate of methamphetamine-positive urine test results significantly declined among participants randomized to mirtazapine vs placebo (risk ratio [RR], 0.67 [95% CI, 0.51-0.87]). Mirtazapine resulted in reductions in positive urine test results at 24 weeks (RR, 0.75 [95% CI, 0.56-1.00]) and 36 weeks (RR, 0.73 [95% CI, 0.57-0.96]) vs placebo. Mean (SD) medication adherence by WisePill dispenser was 38.5% (27.0%) in the mirtazapine group vs 39.5% (26.2%) in the placebo group (P = .77) over 2 to 12 weeks and 28.1% (23.4%) vs 38.5% (27.0%) (P = .59) over 13 to 24 weeks. Changes in sexual risk behaviors were not significantly different by study arm at 12 weeks, but those assigned to receive mirtazapine had fewer sexual partners (RR, 0.52 [95% CI, 0.27-0.97]; P = .04), fewer episodes of condomless anal sex with partners who were serodiscordant (RR, 0.47 [95% CI, 0.23-0.97]; P = .04), and fewer episodes of condomless receptive anal sex with partners who were serodiscordant (RR, 0.37 [95% CI, 0.14-0.93]; P = .04) at week 24. Participants assigned to mirtazapine had net reductions in depressive symptoms (Center for Epidemiologic Studies Depression Scale score, 6.2 [95% CI, 1.3-11.1] points lower; P = .01) and insomnia severity (Athens score, 1.4 [95% CI, 0.1-2.7] points lower; P = .04) at week 24. There were no serious adverse events associated with the study drug.
Conclusions and Relevance
In this expanded replication trial, adding mirtazapine to substance use counseling reduced methamphetamine use and some HIV risk behaviors among cisgender men and transgender women who have sex with men, with benefits extending after treatment despite suboptimal medication adherence.
Trial Registration
ClinicalTrials.gov identifier: NCT01888835.
中文翻译:

米氮平对顺性别男性和与男性发生性关系的跨性别女性甲基苯丙胺使用障碍的影响:安慰剂对照随机临床试验。
重要性 甲基苯丙胺的使用越来越普遍,并与 HIV 传播有关。先前对米氮平的 2a 期研究表明,男男性行为者的甲基苯丙胺使用和性危险行为有所减少。目的确定米氮平治疗甲基苯丙胺使用障碍和降低HIV危险行为的疗效。设计、设置和参与者这项关于米氮平与安慰剂的双盲随机临床试验于 2013 年 8 月至 2017 年 9 月在加利福尼亚州旧金山的一家门诊研究诊所进行。参与者是社区招募的性活跃成年人;顺性别男性、跨性别男性和跨性别女性(1)与男性发生性关系,(2)有甲基苯丙胺使用障碍,(3)积极使用甲基苯丙胺是合格的。参与者被随机分配接受研究药物或安慰剂治疗 24 周,再进行 12 周的随访。数据分析于 2018 年 2 月至 2018 年 6 月进行。每天一次口服米氮平、30 毫克或匹配的安慰剂,持续 24 周,并进行背景咨询。主要结果和措施 12、24 和 36 周(主要结果)和性危险行为(次要结果)的甲基苯丙胺尿检结果呈阳性。评估了睡眠、甲基苯丙胺渴望、依赖严重程度和不良事件。结果 在评估的 241 人中,有 120 人入选(5 名跨性别女性和 115 名顺性别男性)。平均 (SD) 年龄为 43.3 (9.8) 岁;61 人(50.8%)为白人,31 人(25.8%)为非裔美国人,15 人(12.5%)为拉丁裔。总体上完成了 66% (47%) 的平均 (SD) 访问。到第 12 周,在随机分配到米氮平组与安慰剂组的参与者中,甲基苯丙胺尿检阳性率显着下降(风险比 [RR],0.67 [95% CI,0.51-0.87])。与安慰剂相比,米氮平导致 24 周(RR,0.75 [95% CI,0.56-1.00])和 36 周(RR,0.73 [95% CI,0.57-0.96])尿检阳性结果减少。WisePill 分配器的平均 (SD) 药物依从性在 2 至 12 周内米氮平组为 38.5% (27.0%),而安慰剂组为 39.5% (26.2%) (P = .77),28.1% (23.4%) 与13 至 24 周内为 38.5% (27.0%) (P = .59)。研究组在 12 周时性风险行为的变化没有显着差异,但被分配接受米氮平治疗的患者的性伴侣较少(RR,0.52 [95% CI,0.27-0.97];P = .04),没有安全套的次数较少与血清不一致的伴侣肛交(RR,0. 47 [95% CI,0.23-0.97];P = .04),并且在第 24 周时,与血清不一致的伴侣发生的无安全套性肛交次数减少(RR,0.37 [95% CI,0.14-0.93];P = .04)。分配到米氮平组的参与者的抑郁症状(流行病学研究中心抑郁量表评分,低 6.2 [95% CI,1.3-11.1] 分;P = .01)和失眠严重程度(雅典评分,低 1.4 [95% CI,0.1-2.7] 分;P = .04) 在第 24 周。没有与研究药物相关的严重不良事件。结论和相关性 在这项扩大的复制试验中,在物质使用咨询中加入米氮平可减少顺性别男性和男男性行为者跨性别女性中甲基苯丙胺的使用和一些 HIV 危险行为,尽管药物依从性不佳,但治疗后的益处仍然存在。
更新日期:2020-03-05
中文翻译:

米氮平对顺性别男性和与男性发生性关系的跨性别女性甲基苯丙胺使用障碍的影响:安慰剂对照随机临床试验。
重要性 甲基苯丙胺的使用越来越普遍,并与 HIV 传播有关。先前对米氮平的 2a 期研究表明,男男性行为者的甲基苯丙胺使用和性危险行为有所减少。目的确定米氮平治疗甲基苯丙胺使用障碍和降低HIV危险行为的疗效。设计、设置和参与者这项关于米氮平与安慰剂的双盲随机临床试验于 2013 年 8 月至 2017 年 9 月在加利福尼亚州旧金山的一家门诊研究诊所进行。参与者是社区招募的性活跃成年人;顺性别男性、跨性别男性和跨性别女性(1)与男性发生性关系,(2)有甲基苯丙胺使用障碍,(3)积极使用甲基苯丙胺是合格的。参与者被随机分配接受研究药物或安慰剂治疗 24 周,再进行 12 周的随访。数据分析于 2018 年 2 月至 2018 年 6 月进行。每天一次口服米氮平、30 毫克或匹配的安慰剂,持续 24 周,并进行背景咨询。主要结果和措施 12、24 和 36 周(主要结果)和性危险行为(次要结果)的甲基苯丙胺尿检结果呈阳性。评估了睡眠、甲基苯丙胺渴望、依赖严重程度和不良事件。结果 在评估的 241 人中,有 120 人入选(5 名跨性别女性和 115 名顺性别男性)。平均 (SD) 年龄为 43.3 (9.8) 岁;61 人(50.8%)为白人,31 人(25.8%)为非裔美国人,15 人(12.5%)为拉丁裔。总体上完成了 66% (47%) 的平均 (SD) 访问。到第 12 周,在随机分配到米氮平组与安慰剂组的参与者中,甲基苯丙胺尿检阳性率显着下降(风险比 [RR],0.67 [95% CI,0.51-0.87])。与安慰剂相比,米氮平导致 24 周(RR,0.75 [95% CI,0.56-1.00])和 36 周(RR,0.73 [95% CI,0.57-0.96])尿检阳性结果减少。WisePill 分配器的平均 (SD) 药物依从性在 2 至 12 周内米氮平组为 38.5% (27.0%),而安慰剂组为 39.5% (26.2%) (P = .77),28.1% (23.4%) 与13 至 24 周内为 38.5% (27.0%) (P = .59)。研究组在 12 周时性风险行为的变化没有显着差异,但被分配接受米氮平治疗的患者的性伴侣较少(RR,0.52 [95% CI,0.27-0.97];P = .04),没有安全套的次数较少与血清不一致的伴侣肛交(RR,0. 47 [95% CI,0.23-0.97];P = .04),并且在第 24 周时,与血清不一致的伴侣发生的无安全套性肛交次数减少(RR,0.37 [95% CI,0.14-0.93];P = .04)。分配到米氮平组的参与者的抑郁症状(流行病学研究中心抑郁量表评分,低 6.2 [95% CI,1.3-11.1] 分;P = .01)和失眠严重程度(雅典评分,低 1.4 [95% CI,0.1-2.7] 分;P = .04) 在第 24 周。没有与研究药物相关的严重不良事件。结论和相关性 在这项扩大的复制试验中,在物质使用咨询中加入米氮平可减少顺性别男性和男男性行为者跨性别女性中甲基苯丙胺的使用和一些 HIV 危险行为,尽管药物依从性不佳,但治疗后的益处仍然存在。











































 京公网安备 11010802027423号
京公网安备 11010802027423号