当前位置:
X-MOL 学术
›
Cell. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PKC signaling contributes to chromatin decondensation and is required for competence to respond to IL-2 during T cell activation.
Cellular Immunology ( IF 3.7 ) Pub Date : 2019-12-11 , DOI: 10.1016/j.cellimm.2019.104027 Jennifer R Funsten 1 , Keny O Murillo Brizuela 1 , Hayley E Swatzel 1 , Audrey S Ward 1 , Tia A Scott 1 , Sarah M Eikenbusch 1 , Molly C Shields 1 , Jenna L Meredith 1 , Taylor Y Mitchell 1 , Megan L Hanna 1 , Kellie N Bingham 1 , Jason S Rawlings 1
Cellular Immunology ( IF 3.7 ) Pub Date : 2019-12-11 , DOI: 10.1016/j.cellimm.2019.104027 Jennifer R Funsten 1 , Keny O Murillo Brizuela 1 , Hayley E Swatzel 1 , Audrey S Ward 1 , Tia A Scott 1 , Sarah M Eikenbusch 1 , Molly C Shields 1 , Jenna L Meredith 1 , Taylor Y Mitchell 1 , Megan L Hanna 1 , Kellie N Bingham 1 , Jason S Rawlings 1
Affiliation
|
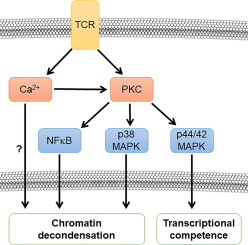
The clonal proliferation of antigen-specific T cells during an immune response critically depends on the differential response to growth factors, such as IL-2. While activated T cells proliferate robustly in response to IL-2 stimulation, naïve (quiescent) T cells are able to ignore the potent effects of growth factors because they possess chromatin that is tightly condensed such that transcription factors, such as STAT5, cannot access DNA. Activation via the T cell receptor (TCR) induces a rapid decondensation of chromatin, permitting STAT5-DNA engagement and ultimately promoting proliferation of only antigen-specific T cells. Previous work demonstrated that the mobilization of intracellular calcium following TCR stimulation is a key event in the decondensation of chromatin. Here we examine PKC-dependent signaling mechanisms to determine their role in activation-induced chromatin decondensation and the subsequent acquisition of competence to respond to IL-2 stimulation. We found that a calcium-dependent PKC contributes to activation-induced chromatin decondensation and that the p38 MAPK and NFκB pathways downstream of PKC each contribute to regulating the proper decondensation of chromatin. Importantly, we found that p44/42 MAPK activity is required for peripheral T cells to gain competence to properly respond to IL-2 stimulation. Our findings shed light on the mechanisms that control the clonal proliferation of antigen-specific peripheral T cells during an immune response.
中文翻译:

PKC 信号传导有助于染色质解浓缩,并且是 T 细胞激活过程中响应 IL-2 的能力所必需的。
免疫反应期间抗原特异性 T 细胞的克隆增殖主要取决于对生长因子(例如 IL-2)的差异反应。虽然活化的 T 细胞响应 IL-2 刺激而强劲增殖,但幼稚(静止)T 细胞能够忽略生长因子的有效作用,因为它们拥有紧密浓缩的染色质,使得转录因子(例如 STAT5)无法接触 DNA 。通过 T 细胞受体 (TCR) 激活可诱导染色质快速解缩,从而允许 STAT5-DNA 结合并最终促进仅抗原特异性 T 细胞的增殖。先前的工作表明,TCR 刺激后细胞内钙的动员是染色质解凝聚的关键事件。在这里,我们检查 PKC 依赖性信号传导机制,以确定它们在激活诱导的染色质解缩以及随后获得对 IL-2 刺激做出反应的能力中的作用。我们发现钙依赖性 PKC 有助于激活诱导的染色质解浓缩,并且 PKC 下游的 p38 MAPK 和 NFκB 通路各自有助于调节染色质的适当解浓缩。重要的是,我们发现 p44/42 MAPK 活性是外周 T 细胞获得正确响应 IL-2 刺激的能力所必需的。我们的研究结果揭示了免疫反应期间控制抗原特异性外周 T 细胞克隆增殖的机制。
更新日期:2019-12-11
中文翻译:

PKC 信号传导有助于染色质解浓缩,并且是 T 细胞激活过程中响应 IL-2 的能力所必需的。
免疫反应期间抗原特异性 T 细胞的克隆增殖主要取决于对生长因子(例如 IL-2)的差异反应。虽然活化的 T 细胞响应 IL-2 刺激而强劲增殖,但幼稚(静止)T 细胞能够忽略生长因子的有效作用,因为它们拥有紧密浓缩的染色质,使得转录因子(例如 STAT5)无法接触 DNA 。通过 T 细胞受体 (TCR) 激活可诱导染色质快速解缩,从而允许 STAT5-DNA 结合并最终促进仅抗原特异性 T 细胞的增殖。先前的工作表明,TCR 刺激后细胞内钙的动员是染色质解凝聚的关键事件。在这里,我们检查 PKC 依赖性信号传导机制,以确定它们在激活诱导的染色质解缩以及随后获得对 IL-2 刺激做出反应的能力中的作用。我们发现钙依赖性 PKC 有助于激活诱导的染色质解浓缩,并且 PKC 下游的 p38 MAPK 和 NFκB 通路各自有助于调节染色质的适当解浓缩。重要的是,我们发现 p44/42 MAPK 活性是外周 T 细胞获得正确响应 IL-2 刺激的能力所必需的。我们的研究结果揭示了免疫反应期间控制抗原特异性外周 T 细胞克隆增殖的机制。









































 京公网安备 11010802027423号
京公网安备 11010802027423号