当前位置:
X-MOL 学术
›
Neurobiol. Aging
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Delivery of exogenous proteins by mesenchymal stem cells attenuates early memory deficits in a murine model of Alzheimer's disease
Neurobiology of Aging ( IF 3.7 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.neurobiolaging.2019.10.012 An Li 1 , Jiayi Zhao 1 , Chongzhu Fan 1 , Lihong Zhu 1 , Cuiqin Huang 1 , Qin Li 1 , Danhui Gan 1 , Caiyan Wen 1 , Mengfei Chen 2 , Daxiang Lu 1
Neurobiology of Aging ( IF 3.7 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.neurobiolaging.2019.10.012 An Li 1 , Jiayi Zhao 1 , Chongzhu Fan 1 , Lihong Zhu 1 , Cuiqin Huang 1 , Qin Li 1 , Danhui Gan 1 , Caiyan Wen 1 , Mengfei Chen 2 , Daxiang Lu 1
Affiliation
|
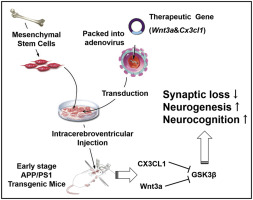
A promising intervention for Alzheimer's disease (AD) would ideally target key pathological factors that are involved in AD pathogenesis. Soluble factors produced by engrafted mesenchymal stem cells (MSCs) mediate potential therapeutic effects in AD. However, these therapeutic benefits are largely hampered by the limited paracrine capacity of MSCs. In this study, we used adenovirus-mediated gene transduction of bone marrow MSCs to deliver exogenous proteins into the brain of APPswe/PSEN1dE9 (APP/PS1) mice in the early stage of impairment. We observed that engrafted MSCs carrying exogenous (C-X3-C motif) ligand 1 (CX3CL1) alone reduced the production of the inflammatory cytokine TNF-ɑ and improved synapse-related protein expression but not cognitive function. Transplantation of MSCs carrying CX3CL1 and Wnt3a (CX3CL1-Wnt3a-MSC) significantly attenuated the learning and memory impairment when compared with a control group. The improvement of neurobehavioral functions in APP/PS1 mice treated with CX3CL1-Wnt3a-MSC was related to the inhibition of microglial neurotoxicity and promotion of hippocampal neurogenesis. Transplantation of CX3CL1-Wnt3a-MSC also regulated phosphoinositide 3-kinase/activated protein kinase B (PI3K/AKT) signaling to inhibit the activity of glycogen synthase kinase 3 beta (GSK3β). Taken together, these results indicate that the delivery of exogenous proteins via MSCs can modulate microglial function and enhance neurogenesis, thereby providing new insights into AD intervention.
中文翻译:

间充质干细胞传递外源蛋白可减轻阿尔茨海默病小鼠模型的早期记忆缺陷
阿尔茨海默病 (AD) 的有希望的干预措施将理想地针对 AD 发病机制中涉及的关键病理因素。由移植的间充质干细胞 (MSC) 产生的可溶性因子介导 AD 的潜在治疗效果。然而,这些治疗益处在很大程度上受到 MSC 有限的旁分泌能力的阻碍。在这项研究中,我们使用腺病毒介导的骨髓间充质干细胞基因转导将外源蛋白递送到损伤早期的 APPswe/PSEN1dE9 (APP/PS1) 小鼠的大脑中。我们观察到,单独携带外源性(C-X3-C 基序)配体 1(CX3CL1)的移植 MSC 减少了炎性细胞因子 TNF-ɑ 的产生,并改善了突触相关蛋白的表达,但不改善认知功能。与对照组相比,移植携带 CX3CL1 和 Wnt3a(CX3CL1-Wnt3a-MSC)的 MSC 显着减轻了学习和记忆障碍。CX3CL1-Wnt3a-MSC治疗APP/PS1小鼠神经行为功能的改善与抑制小胶质细胞神经毒性和促进海马神经发生有关。CX3CL1-Wnt3a-MSC 的移植还调节磷酸肌醇 3-激酶/活化蛋白激酶 B (PI3K/AKT) 信号传导以抑制糖原合酶激酶 3β (GSK3β) 的活性。综上所述,这些结果表明通过 MSCs 传递外源蛋白可以调节小胶质细胞功能并增强神经发生,从而为 AD 干预提供新的见解。CX3CL1-Wnt3a-MSC治疗APP/PS1小鼠神经行为功能的改善与抑制小胶质细胞神经毒性和促进海马神经发生有关。CX3CL1-Wnt3a-MSC 的移植还调节磷酸肌醇 3-激酶/活化蛋白激酶 B (PI3K/AKT) 信号传导以抑制糖原合酶激酶 3β (GSK3β) 的活性。综上所述,这些结果表明通过 MSCs 传递外源蛋白可以调节小胶质细胞功能并增强神经发生,从而为 AD 干预提供新的见解。CX3CL1-Wnt3a-MSC治疗APP/PS1小鼠神经行为功能的改善与抑制小胶质细胞神经毒性和促进海马神经发生有关。CX3CL1-Wnt3a-MSC 的移植还调节磷酸肌醇 3-激酶/活化蛋白激酶 B (PI3K/AKT) 信号传导以抑制糖原合酶激酶 3β (GSK3β) 的活性。综上所述,这些结果表明通过 MSCs 传递外源蛋白可以调节小胶质细胞功能并增强神经发生,从而为 AD 干预提供新的见解。CX3CL1-Wnt3a-MSC 的移植还调节磷酸肌醇 3-激酶/活化蛋白激酶 B (PI3K/AKT) 信号传导以抑制糖原合酶激酶 3β (GSK3β) 的活性。综上所述,这些结果表明,通过 MSCs 传递外源蛋白可以调节小胶质细胞功能并增强神经发生,从而为 AD 干预提供新的见解。CX3CL1-Wnt3a-MSC 的移植还调节磷酸肌醇 3-激酶/活化蛋白激酶 B (PI3K/AKT) 信号传导以抑制糖原合酶激酶 3β (GSK3β) 的活性。综上所述,这些结果表明通过 MSCs 传递外源蛋白可以调节小胶质细胞功能并增强神经发生,从而为 AD 干预提供新的见解。
更新日期:2020-02-01
中文翻译:

间充质干细胞传递外源蛋白可减轻阿尔茨海默病小鼠模型的早期记忆缺陷
阿尔茨海默病 (AD) 的有希望的干预措施将理想地针对 AD 发病机制中涉及的关键病理因素。由移植的间充质干细胞 (MSC) 产生的可溶性因子介导 AD 的潜在治疗效果。然而,这些治疗益处在很大程度上受到 MSC 有限的旁分泌能力的阻碍。在这项研究中,我们使用腺病毒介导的骨髓间充质干细胞基因转导将外源蛋白递送到损伤早期的 APPswe/PSEN1dE9 (APP/PS1) 小鼠的大脑中。我们观察到,单独携带外源性(C-X3-C 基序)配体 1(CX3CL1)的移植 MSC 减少了炎性细胞因子 TNF-ɑ 的产生,并改善了突触相关蛋白的表达,但不改善认知功能。与对照组相比,移植携带 CX3CL1 和 Wnt3a(CX3CL1-Wnt3a-MSC)的 MSC 显着减轻了学习和记忆障碍。CX3CL1-Wnt3a-MSC治疗APP/PS1小鼠神经行为功能的改善与抑制小胶质细胞神经毒性和促进海马神经发生有关。CX3CL1-Wnt3a-MSC 的移植还调节磷酸肌醇 3-激酶/活化蛋白激酶 B (PI3K/AKT) 信号传导以抑制糖原合酶激酶 3β (GSK3β) 的活性。综上所述,这些结果表明通过 MSCs 传递外源蛋白可以调节小胶质细胞功能并增强神经发生,从而为 AD 干预提供新的见解。CX3CL1-Wnt3a-MSC治疗APP/PS1小鼠神经行为功能的改善与抑制小胶质细胞神经毒性和促进海马神经发生有关。CX3CL1-Wnt3a-MSC 的移植还调节磷酸肌醇 3-激酶/活化蛋白激酶 B (PI3K/AKT) 信号传导以抑制糖原合酶激酶 3β (GSK3β) 的活性。综上所述,这些结果表明通过 MSCs 传递外源蛋白可以调节小胶质细胞功能并增强神经发生,从而为 AD 干预提供新的见解。CX3CL1-Wnt3a-MSC治疗APP/PS1小鼠神经行为功能的改善与抑制小胶质细胞神经毒性和促进海马神经发生有关。CX3CL1-Wnt3a-MSC 的移植还调节磷酸肌醇 3-激酶/活化蛋白激酶 B (PI3K/AKT) 信号传导以抑制糖原合酶激酶 3β (GSK3β) 的活性。综上所述,这些结果表明通过 MSCs 传递外源蛋白可以调节小胶质细胞功能并增强神经发生,从而为 AD 干预提供新的见解。CX3CL1-Wnt3a-MSC 的移植还调节磷酸肌醇 3-激酶/活化蛋白激酶 B (PI3K/AKT) 信号传导以抑制糖原合酶激酶 3β (GSK3β) 的活性。综上所述,这些结果表明,通过 MSCs 传递外源蛋白可以调节小胶质细胞功能并增强神经发生,从而为 AD 干预提供新的见解。CX3CL1-Wnt3a-MSC 的移植还调节磷酸肌醇 3-激酶/活化蛋白激酶 B (PI3K/AKT) 信号传导以抑制糖原合酶激酶 3β (GSK3β) 的活性。综上所述,这些结果表明通过 MSCs 传递外源蛋白可以调节小胶质细胞功能并增强神经发生,从而为 AD 干预提供新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号