Journal of Pharmaceutical Analysis ( IF 6.1 ) Pub Date : 2019-11-28 , DOI: 10.1016/j.jpha.2019.11.006 Olga Begou 1, 2, 3 , Kathrin Drabert 1 , Georgios Theodoridis 2, 3 , Dimitrios Tsikas 1
|
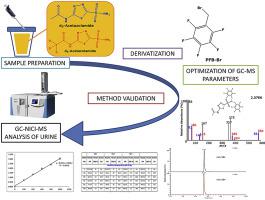
Acetazolamide (molecular mass (MM), 222) belongs to the class of sulfonamides (R-SO2-NH2) and is one of the strongest pharmacological inhibitors of carbonic anhydrase activity. Acetazolamide is excreted unchanged in the urine. Here, we report on the development, validation and biomedical application of a stable-isotope dilution GC-MS method for the reliable quantitative determination of acetazolamide in human urine. The method is based on evaporation to dryness of 50 μL urine aliquots, base-catalyzed derivatization of acetazolamide (d0-AZM) and its internal standard [acetylo-2H3]acetazolamide (d3-AZM) in 30 vol% pentafluorobenzyl (PFB) bromide in acetonitrile (60 min, 30 °C), reconstitution in toluene (200 μL) and injection of 1-μL aliquots. The negative-ion chemical ionization (NICI) mass spectra (methane) of the PFB derivatives contained several intense ions including [M]‒ at m/z 581 for d0-AZM and m/z 584 for d3-AZM, suggesting derivatization of their sulfonamide groups to form N,N-dipentafluorobenzyl derivatives (R-SO2-N(PFB)2), i.e., d0-AZM-(PFB)2 and d3-AZM-(PFB)2, respectively. Quantification was performed by selected-ion monitoring of m/z 581 and 83 for d0-AZM-(PFB)2 and m/z 584 and 86 for d3-AZM-(PFB)2. The limits of detection and quantitation of the method were determined to be 300 fmol (67 pg) and 1 μM of acetazolamide, respectively. Intra- and inter-assay precision and accuracy for acetazolamide in human urine samples in pharmacologically relevant concentration ranges were determined to be 0.3%–4.2% and 95.3%–109%, respectively. The method was applied to measure urinary acetazolamide excretion after ingestion of a 250 mg acetazolamide-containing tablet (Acemit®) by a healthy volunteer. Among other tested sulfonamide drugs, methazolamide (MM, 236) was also found to form a N,N-dipentafluorobenzyl derivative, whereas dorzolamide (MM, 324) was hardly detectable. No GC-MS peaks were obtained from the PFB bromide derivatization of hydrochlorothiazide (MM, 298), xipamide (MM, 355), indapamide and metholazone (MM, 366 each) or brinzolamide (MM, 384). We demonstrate for the first time that sulfonamide drugs can be derivatized with PFB bromide and quantitated by GC-MS. Sulfonamides with MM larger than 236 are likely to be derivatized by PFB bromide but to lack thermal stability.
中文翻译:

GC-NICI-MS 分析乙酰唑胺和其他磺胺 (R-SO2-NH2) 药物作为五氟苄基衍生物 [R-SO2-N(PFB)2] 并定量人尿中的药理学乙酰唑胺。
乙酰唑胺(分子质量(MM),222)属于磺胺类(R-SO 2 -NH 2),是碳酸酐酶活性最强的药理学抑制剂之一。乙酰唑胺以原形从尿中排出。在这里,我们报告了稳定同位素稀释 GC-MS 方法的开发、验证和生物医学应用,用于可靠地定量测定人尿中乙酰唑胺。该方法基于蒸发至干 50 μL 尿液等分试样、乙酰唑胺 (d 0 -AZM ) 及其内标物 [乙酰基- 2 H 3 ] 乙酰唑胺 (d 3-AZM) 在 30 vol% 五氟苄基 (PFB) 溴化物的乙腈溶液中(60 分钟,30 °C),在甲苯 (200 μL) 中复溶,并注入 1-μL 等分试样。PFB 衍生物的负离子化学电离 (NICI) 质谱(甲烷)包含几个强离子,包括 [M] – d 0 -AZM的m/z 581和 d 3 -AZM 的m/z 584 ,表明衍生化它们的磺酰胺基团形成N,N-二五氟苄基衍生物(R-SO 2 -N(PFB) 2 ),即d 0 -AZM-(PFB) 2和d 3 -AZM-(PFB) 2, 分别。通过选择离子监测 d 0 -AZM-(PFB) 2 的m / z 581 和 83 以及 d 3 -AZM-(PFB) 2的m / z 584和86 进行定量. 该方法的检测限和定量限分别确定为 300 fmol (67 pg) 和 1 μM 乙酰唑胺。在药理学相关浓度范围内,人尿样中乙酰唑胺的测定内和测定间精密度和准确度分别确定为 0.3%–4.2% 和 95.3%–109%。该方法用于测量健康志愿者摄入 250 mg 含乙酰唑胺片剂 (Acemit®) 后的尿乙酰唑胺排泄量。在其他测试过的磺胺类药物中,甲唑胺 (MM, 236) 也被发现可形成N,N-二五氟苄基衍生物,而多佐胺(MM,324)几乎检测不到。氢氯噻嗪 (MM, 298)、西帕胺 (MM, 355)、吲达帕胺和美乐酮 (MM, 各 366) 或布林佐胺 (MM, 384) 的 PFB 溴化物衍生化未获得 GC-MS 峰。我们首次证明磺胺类药物可以用 PFB 溴化物衍生并通过 GC-MS 进行定量。MM 大于 236 的磺胺类药物很可能被 PFB 溴化物衍生,但缺乏热稳定性。









































 京公网安备 11010802027423号
京公网安备 11010802027423号