Journal of Pharmaceutical Analysis ( IF 8.8 ) Pub Date : 2019-09-17 , DOI: 10.1016/j.jpha.2019.09.002 Fengting Ou , Ying Zhou , Jinxiu Lei , Su Zeng , Fuhai Wu , Ning Zhang , Lushan Yu
|
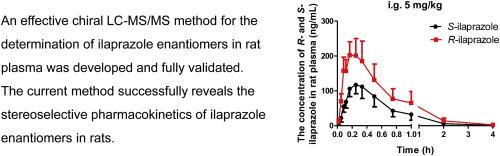
In Korea and China, ilaprazole is a widely used proton pump inhibitor in the treatment of gastric ulcers. In this study, a specific and sensitive LC-MS/MS method has been developed and validated for the quantification of ilaprazole enantiomers in the rat plasma, using R-lansoprazole as the internal standard. The enantioseparation was achieved on a CHIRALPAK AS-RH column (4.6 mm × 150 mm, i.d. 5 μm), with a mobile phase composed of 10 mM ammonium acetate aqueous solution and acetonitrile (60:40, V/V), at a flow-rate of 0.5 mL/min. The method was validated over the concentration range of 0.5–300 ng/mL for both, R- and S -ilaprazole. The lower limit of quantification was 0.5 ng/mL for both enantiomers. The relative standard deviation (RSD) of intra- and inter-day precision of R-ilaprazole and S-ilaprazole was less than 10.9%, and the relative error accuracy (RE) ranged from −0.5%–2.0%. Finally, the method was successfully evaluated in rats in a stereoselective pharmacokinetic study of the ilaprazole racemate.



























 京公网安备 11010802027423号
京公网安备 11010802027423号