Journal of Energy Chemistry ( IF 14.0 ) Pub Date : 2019-10-31 , DOI: 10.1016/j.jechem.2019.10.014 Pei Yu , Han Wu , Jianping Guo , Peikun Wang , Fei Chang , Wenbo Gao , Weijin Zhang , Lin Liu , Ping Chen
|
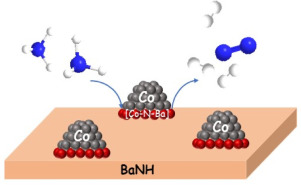
Development of active and non-noble metal-based catalyst for H2 production via NH3 decomposition is crucial for the implementation of NH3 as a H2 carrier. Co-based catalysts have received increasing attention because of its high intrinsic activity and moderate cost. In this work, we examined the effect of BaNH, CaNH and Mg3N2 on the catalytic activity of Co in the NH3 decomposition reaction. The H2 formation rate ranks the order as Co-BaNH > Co-CaNH > Co-Mg3N2 ≈ Co/CNTs within a reaction temperature range of 300–550 °C. It is worth pointing out that the H2 formation rate of Co-BaNH at 500 °C reaches 20 mmolH2 gcat–1 min–1, which is comparable to those of the active Ru/Al2O3 (ca. 17 mmolH2 gcat–1 min–1) and Ru/AC (21 mmolH2 gcat–1 min–1) catalysts under the similar reaction conditions. In-depth research shows that Co-BaNH exhibits an obviously higher intrinsic activity and much lower Ea (46.2 kJ mol–1) than other Co-based catalysts, suggesting that BaNH may play a different role from CaNH, Mg3N2 and CNTs during the catalytic process. Combined results of XRD, Ar-TPD and XAS show that a [Co–N–Ba]-like intermediate species is likely formed at the interface of Co metal and BaNH, which may lead to a more energy-efficient reaction pathway than that of neat Co metal for NH3 decomposition.
中文翻译:

BaNH,CaNH,Mg 3 N 2对Co在NH 3分解催化中活性的影响
通过NH 3分解生产H 2的活性和非贵金属基催化剂的开发对于将NH 3用作H 2载体至关重要。钴基催化剂因其高内在活性和适中的成本而受到越来越多的关注。在这项工作中,我们研究了BaNH,CaNH和Mg 3 N 2对Co在NH 3分解反应中的催化活性的影响。为H 2形成速率行列的顺序为联合迪班>联合CANH>共同的Mg 3 Ñ 2 ≈的Co /反应温度范围300-550℃的范围内的CNT。值得指出的是H 2500°C时Co-BaNH的生成速率达到20 mmol H2 g cat –1 min –1,与活性Ru / Al 2 O 3的形成速率相当(约17 mmol H2 g cat –1 min –1)。和Ru / AC(21 mmol H2 g cat –1 min –1)催化剂在相似的反应条件下。深入的研究表明,Co-BaNH的内在活性明显高于其他Co-基催化剂,而E a(46.2 kJ mol –1)则要低得多,这表明BaNH可能起与CaNH,Mg不同的作用。3 N 2和碳纳米管在催化过程中。XRD,Ar-TPD和XAS的综合结果表明,在Co金属和BaNH的界面上可能形成[Co–N–Ba]状中间物,这可能导致比Co2的反应更节能的反应路径。用于NH 3分解的纯Co金属。









































 京公网安备 11010802027423号
京公网安备 11010802027423号