当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heteroarylamide smoothened inhibitors: Discovery of N-[2,4-dimethyl-5-(1-methylimidazol-4-yl)phenyl]-4-(2-pyridylmethoxy)benzamide (AZD8542) and N-[5-(1H-imidazol-2-yl)-2,4-dimethyl-phenyl]-4-(2- pyridylmethoxy)benzamide (AZD7254).
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-12-11 , DOI: 10.1016/j.bmc.2019.115227 Bin Yang 1 , Alexander W Hird 1 , Michael S Bodnarchuk 2 , Xiaolan Zheng 1 , Les Dakin 1 , Qibin Su 1 , Kevin Daly 1 , Robert Godin 3 , Maureen M Hattersley 3 , Patrick Brassil 4 , Sean Redmond 5 , Daniel John Russell 1 , James W Janetka 1
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2019-12-11 , DOI: 10.1016/j.bmc.2019.115227 Bin Yang 1 , Alexander W Hird 1 , Michael S Bodnarchuk 2 , Xiaolan Zheng 1 , Les Dakin 1 , Qibin Su 1 , Kevin Daly 1 , Robert Godin 3 , Maureen M Hattersley 3 , Patrick Brassil 4 , Sean Redmond 5 , Daniel John Russell 1 , James W Janetka 1
Affiliation
|
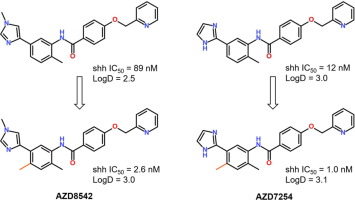
Aberrant hedgehog (Hh) pathway signaling is implicated in multiple cancer types and targeting the Smoothened (SMO) receptor, a key protein of the Hh pathway, has proven effective in treating metastasized basal cell carcinoma. Our lead optimization effort focused on a series of heteroarylamides. We observed that a methyl substitution ortho to the heteroaryl groups on an aniline core significantly improved the potency of this series of compounds. These findings predated the availability of SMO crystal structure in 2013. Here we retrospectively applied quantum mechanics calculations to demonstrate the o-Me substitution favors the bioactive conformation by inducing a dihedral twist between the heteroaryl rings and the core aniline. The o-Me also makes favorable hydrophobic interactions with key residue side chains in the binding pocket. From this effort, two compounds (AZD8542 and AZD7254) showed excellent pharmacokinetics across multiple preclinical species and demonstrated in vivo activity in abrogating the Hh paracrine pathway as well as anti- tumor effects.
中文翻译:

杂芳基酰胺平滑抑制剂:N- [2,4-二甲基-5-(1-甲基咪唑-4-基)苯基] -4-(2-吡啶基甲氧基)苯甲酰胺(AZD8542)和N- [5-(1H-咪唑) -2-基)-2,4-二甲基-苯基] -4-(2-吡啶基甲氧基)苯甲酰胺(AZD7254)。
异常的刺猬(Hh)途径信号传导涉及多种癌症类型,靶向Hh途径的关键蛋白“ Smoothened(SMO)”受体已被证明可有效治疗转移的基底细胞癌。我们的主要优化工作集中在一系列杂芳基酰胺上。我们观察到苯胺核心上杂芳基的邻位甲基取代显着提高了该系列化合物的效价。这些发现早于2013年SMO晶体结构的可用性。在这里,我们回顾性地应用了量子力学计算,以证明o-Me取代通过诱导杂芳基环与核心苯胺之间的二面角扭曲而有利于生物活性构象。o-Me还可以与结合袋中的关键残基侧链形成有利的疏水相互作用。
更新日期:2019-12-11
中文翻译:

杂芳基酰胺平滑抑制剂:N- [2,4-二甲基-5-(1-甲基咪唑-4-基)苯基] -4-(2-吡啶基甲氧基)苯甲酰胺(AZD8542)和N- [5-(1H-咪唑) -2-基)-2,4-二甲基-苯基] -4-(2-吡啶基甲氧基)苯甲酰胺(AZD7254)。
异常的刺猬(Hh)途径信号传导涉及多种癌症类型,靶向Hh途径的关键蛋白“ Smoothened(SMO)”受体已被证明可有效治疗转移的基底细胞癌。我们的主要优化工作集中在一系列杂芳基酰胺上。我们观察到苯胺核心上杂芳基的邻位甲基取代显着提高了该系列化合物的效价。这些发现早于2013年SMO晶体结构的可用性。在这里,我们回顾性地应用了量子力学计算,以证明o-Me取代通过诱导杂芳基环与核心苯胺之间的二面角扭曲而有利于生物活性构象。o-Me还可以与结合袋中的关键残基侧链形成有利的疏水相互作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号