当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total synthesis of exigurin: the Ugi reaction in a hypothetical biosynthesis of natural products.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-06 , DOI: 10.1039/c9ob02249j Seijiro Hosokawa 1 , Keisuke Nakanishi 1 , Yutaro Udagawa 1 , Mitsutoshi Maeda 2 , Seiya Sato 2 , Keiji Nakano 2 , Toshiya Masuda 3 , Yoshiyasu Ichikawa 2
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-01-06 , DOI: 10.1039/c9ob02249j Seijiro Hosokawa 1 , Keisuke Nakanishi 1 , Yutaro Udagawa 1 , Mitsutoshi Maeda 2 , Seiya Sato 2 , Keiji Nakano 2 , Toshiya Masuda 3 , Yoshiyasu Ichikawa 2
Affiliation
|
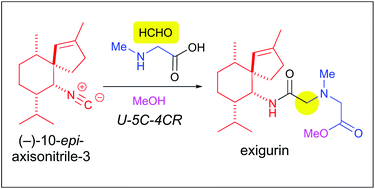
The first total synthesis of a marine natural product, exigurin, has been accomplished in 13 steps starting from (+)-menthone. The key intermediate (-)-10-epi-axisonitrile-3 was prepared by stereoselective intramolecular cyclopropanation followed by a cyclopropane ring opening reaction by the azide anion. The bioinspired Ugi reaction of (-)-10-epi-axisonitrile-3, formaldehyde, sarcosine and methanol successfully constructed the target exigurin in which its terpene and amino acid units were linked through an amide bond.
中文翻译:

Exigurin的全合成:假设的天然产物生物合成中的Ugi反应。
从(+)-薄荷酮开始,海洋天然产物exigurin的第一个全合成已完成了13个步骤。通过立体选择性分子内环丙烷化,然后通过叠氮化物阴离子进行环丙烷开环反应,制备关键的中间体(-)-10-表轴腈-3。(-)-10-epi-axisonitrile-3,甲醛,肌氨酸和甲醇的生物启发性Ugi反应成功构建了目标exigurin,其萜烯和氨基酸单元通过酰胺键连接。
更新日期:2020-02-10
中文翻译:

Exigurin的全合成:假设的天然产物生物合成中的Ugi反应。
从(+)-薄荷酮开始,海洋天然产物exigurin的第一个全合成已完成了13个步骤。通过立体选择性分子内环丙烷化,然后通过叠氮化物阴离子进行环丙烷开环反应,制备关键的中间体(-)-10-表轴腈-3。(-)-10-epi-axisonitrile-3,甲醛,肌氨酸和甲醇的生物启发性Ugi反应成功构建了目标exigurin,其萜烯和氨基酸单元通过酰胺键连接。











































 京公网安备 11010802027423号
京公网安备 11010802027423号