当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalysis, kinetics and mechanisms of organo-iridium enantioselective hydrogenation-reduction
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2019/12/11 , DOI: 10.1039/c9cy02147g Joseph M. Mwansa 1, 2, 3, 4 , Michael I. Page 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2019/12/11 , DOI: 10.1039/c9cy02147g Joseph M. Mwansa 1, 2, 3, 4 , Michael I. Page 1, 2, 3, 4
Affiliation
|
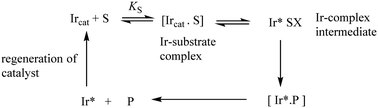
The synthesis of chiral molecules is of great importance to the pharmaceutical, agrochemical, flavour and fragrance industries. The use of organo-iridium complexes has gained a reputation for its great utility in key enantioselective synthetic procedures. Prime examples include the catalytic reduction of carbonyls and imines; iridium-catalysed allylic substitution and catalysed enantioselective hydrogenation of unsaturated carboxylic acids. Important aspects in these processes are the reaction conditions such as the catalyst loading, metal-ion ligands, the substrate, solvent and the reaction times-all of which can affect the degree of enantioselectivity. Understanding the mechanisms of these hydrogenation/reduction reactions through kinetic and other related studies makes a vital contribution to improving catalytic efficiency.
中文翻译:

有机铱对映选择性加氢还原的催化,动力学和机理
手性分子的合成对制药,农用化学,香料和香料工业非常重要。有机铱配合物的使用因其在关键对映选择性合成过程中的巨大实用性而赢得了声誉。主要的例子包括羰基和亚胺的催化还原。铱催化的烯丙基取代和不饱和羧酸的催化对映选择性氢化。这些方法中的重要方面是反应条件,例如催化剂负载量,金属离子配体,底物,溶剂和反应时间,所有这些都会影响对映选择性。通过动力学和其他相关研究了解这些氢化/还原反应的机理,对提高催化效率做出了重要贡献。
更新日期:2020-02-13
中文翻译:

有机铱对映选择性加氢还原的催化,动力学和机理
手性分子的合成对制药,农用化学,香料和香料工业非常重要。有机铱配合物的使用因其在关键对映选择性合成过程中的巨大实用性而赢得了声誉。主要的例子包括羰基和亚胺的催化还原。铱催化的烯丙基取代和不饱和羧酸的催化对映选择性氢化。这些方法中的重要方面是反应条件,例如催化剂负载量,金属离子配体,底物,溶剂和反应时间,所有这些都会影响对映选择性。通过动力学和其他相关研究了解这些氢化/还原反应的机理,对提高催化效率做出了重要贡献。











































 京公网安备 11010802027423号
京公网安备 11010802027423号