当前位置:
X-MOL 学术
›
PLOS Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BMPR2 acts as a gatekeeper to protect endothelial cells from increased TGFβ responses and altered cell mechanics.
PLOS Biology ( IF 7.8 ) Pub Date : 2019-12-11 , DOI: 10.1371/journal.pbio.3000557 Christian Hiepen 1 , Jerome Jatzlau 1, 2 , Susanne Hildebrandt 1, 2 , Branka Kampfrath 1 , Melis Goktas 3 , Arunima Murgai 1, 2, 4 , Jose Luis Cuellar Camacho 1 , Rainer Haag 1 , Clemens Ruppert 5 , Gerhard Sengle 6 , Elisabetta Ada Cavalcanti-Adam 7 , Kerstin G Blank 3 , Petra Knaus 1
PLOS Biology ( IF 7.8 ) Pub Date : 2019-12-11 , DOI: 10.1371/journal.pbio.3000557 Christian Hiepen 1 , Jerome Jatzlau 1, 2 , Susanne Hildebrandt 1, 2 , Branka Kampfrath 1 , Melis Goktas 3 , Arunima Murgai 1, 2, 4 , Jose Luis Cuellar Camacho 1 , Rainer Haag 1 , Clemens Ruppert 5 , Gerhard Sengle 6 , Elisabetta Ada Cavalcanti-Adam 7 , Kerstin G Blank 3 , Petra Knaus 1
Affiliation
|
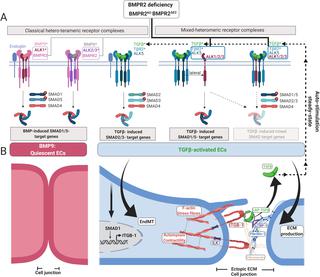
Balanced transforming growth factor-beta (TGFβ)/bone morphogenetic protein (BMP)-signaling is essential for tissue formation and homeostasis. While gain in TGFβ signaling is often found in diseases, the underlying cellular mechanisms remain poorly defined. Here we show that the receptor BMP type 2 (BMPR2) serves as a central gatekeeper of this balance, highlighted by its deregulation in diseases such as pulmonary arterial hypertension (PAH). We show that BMPR2 deficiency in endothelial cells (ECs) does not abolish pan-BMP-SMAD1/5 responses but instead favors the formation of mixed-heteromeric receptor complexes comprising BMPR1/TGFβR1/TGFβR2 that enable enhanced cellular responses toward TGFβ. These include canonical TGFβ-SMAD2/3 and lateral TGFβ-SMAD1/5 signaling as well as formation of mixed SMAD complexes. Moreover, BMPR2-deficient cells express genes indicative of altered biophysical properties, including up-regulation of extracellular matrix (ECM) proteins such as fibrillin-1 (FBN1) and of integrins. As such, we identified accumulation of ectopic FBN1 fibers remodeled with fibronectin (FN) in junctions of BMPR2-deficient ECs. Ectopic FBN1 deposits were also found in proximity to contractile intimal cells in pulmonary artery lesions of BMPR2-deficient heritable PAH (HPAH) patients. In BMPR2-deficient cells, we show that ectopic FBN1 is accompanied by active β1-integrin highly abundant in integrin-linked kinase (ILK) mechano-complexes at cell junctions. Increased integrin-dependent adhesion, spreading, and actomyosin-dependent contractility facilitates the retrieval of active TGFβ from its latent fibrillin-bound depots. We propose that loss of BMPR2 favors endothelial-to-mesenchymal transition (EndMT) allowing cells of myo-fibroblastic character to create a vicious feed-forward process leading to hyperactivated TGFβ signaling. In summary, our findings highlight a crucial role for BMPR2 as a gatekeeper of endothelial homeostasis protecting cells from increased TGFβ responses and integrin-mediated mechano-transduction.
中文翻译:

BMPR2 作为看门人,保护内皮细胞免受 TGFβ 反应增加和细胞力学改变的影响。
平衡的转化生长因子-β (TGFβ)/骨形态发生蛋白 (BMP) 信号传导对于组织形成和稳态至关重要。虽然疾病中经常发现 TGFβ 信号传导增强,但其潜在的细胞机制仍不清楚。在这里,我们发现 BMP 2 型受体 (BMPR2) 是这种平衡的中央看门人,这一点在肺动脉高压 (PAH) 等疾病中的失调中得到了凸显。我们发现内皮细胞(EC)中的 BMPR2 缺陷不会消除泛 BMP-SMAD1/5 反应,而是有利于形成包含 BMPR1/TGFβR1/TGFβR2 的混合异聚受体复合物,从而增强细胞对 TGFβ 的反应。这些包括经典 TGFβ-SMAD2/3 和横向 TGFβ-SMAD1/5 信号传导以及混合 SMAD 复合物的形成。此外,BMPR2缺陷细胞表达的基因表明生物物理特性发生改变,包括细胞外基质(ECM)蛋白如纤维蛋白-1(FBN1)和整合素的上调。因此,我们在 BMPR2 缺陷的 EC 的连接处发现了用纤连蛋白 (FN) 重塑的异位 FBN1 纤维的积累。在 BMPR2 缺陷型遗传性 PAH (HPAH) 患者肺动脉病变的收缩性内膜细胞附近也发现了异位 FBN1 沉积物。在 BMPR2 缺陷细胞中,我们发现异位 FBN1 伴随着细胞连接处整合素连接激酶 (ILK) 机械复合物中高度丰富的活性 β1-整合素。整合素依赖性粘附、扩散和肌动球蛋白依赖性收缩性的增加有助于从其潜在的原纤维蛋白结合库中回收活性 TGFβ。我们认为,BMPR2 的缺失有利于内皮向间质转化 (EndMT),从而使肌成纤维细胞特征的细胞产生恶性前馈过程,导致 TGFβ 信号传导过度激活。总之,我们的研究结果强调了 BMPR2 作为内皮稳态守门人的关键作用,保护细胞免受 TGFβ 反应增加和整合素介导的机械转导的影响。
更新日期:2019-12-11
中文翻译:

BMPR2 作为看门人,保护内皮细胞免受 TGFβ 反应增加和细胞力学改变的影响。
平衡的转化生长因子-β (TGFβ)/骨形态发生蛋白 (BMP) 信号传导对于组织形成和稳态至关重要。虽然疾病中经常发现 TGFβ 信号传导增强,但其潜在的细胞机制仍不清楚。在这里,我们发现 BMP 2 型受体 (BMPR2) 是这种平衡的中央看门人,这一点在肺动脉高压 (PAH) 等疾病中的失调中得到了凸显。我们发现内皮细胞(EC)中的 BMPR2 缺陷不会消除泛 BMP-SMAD1/5 反应,而是有利于形成包含 BMPR1/TGFβR1/TGFβR2 的混合异聚受体复合物,从而增强细胞对 TGFβ 的反应。这些包括经典 TGFβ-SMAD2/3 和横向 TGFβ-SMAD1/5 信号传导以及混合 SMAD 复合物的形成。此外,BMPR2缺陷细胞表达的基因表明生物物理特性发生改变,包括细胞外基质(ECM)蛋白如纤维蛋白-1(FBN1)和整合素的上调。因此,我们在 BMPR2 缺陷的 EC 的连接处发现了用纤连蛋白 (FN) 重塑的异位 FBN1 纤维的积累。在 BMPR2 缺陷型遗传性 PAH (HPAH) 患者肺动脉病变的收缩性内膜细胞附近也发现了异位 FBN1 沉积物。在 BMPR2 缺陷细胞中,我们发现异位 FBN1 伴随着细胞连接处整合素连接激酶 (ILK) 机械复合物中高度丰富的活性 β1-整合素。整合素依赖性粘附、扩散和肌动球蛋白依赖性收缩性的增加有助于从其潜在的原纤维蛋白结合库中回收活性 TGFβ。我们认为,BMPR2 的缺失有利于内皮向间质转化 (EndMT),从而使肌成纤维细胞特征的细胞产生恶性前馈过程,导致 TGFβ 信号传导过度激活。总之,我们的研究结果强调了 BMPR2 作为内皮稳态守门人的关键作用,保护细胞免受 TGFβ 反应增加和整合素介导的机械转导的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号