当前位置:
X-MOL 学术
›
PLOS Pathog.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deep sequence analysis of HIV adaptation following vertical transmission reveals the impact of immune pressure on the evolution of HIV.
PLoS Pathogens ( IF 5.5 ) Pub Date : 2019-12-10 , DOI: 10.1371/journal.ppat.1008177 Jennifer Currenti 1 , Abha Chopra 2 , Mina John 2, 3 , Shay Leary 2 , Elizabeth McKinnon 2 , Eric Alves 1 , Mark Pilkinton 4 , Rita Smith 4 , Louise Barnett 4 , Wyatt J McDonnell 4 , Michaela Lucas 5 , Francine Noel 6 , Simon Mallal 2, 4 , Joseph A Conrad 7 , Spyros A Kalams 4 , Silvana Gaudieri 1, 2, 4
PLoS Pathogens ( IF 5.5 ) Pub Date : 2019-12-10 , DOI: 10.1371/journal.ppat.1008177 Jennifer Currenti 1 , Abha Chopra 2 , Mina John 2, 3 , Shay Leary 2 , Elizabeth McKinnon 2 , Eric Alves 1 , Mark Pilkinton 4 , Rita Smith 4 , Louise Barnett 4 , Wyatt J McDonnell 4 , Michaela Lucas 5 , Francine Noel 6 , Simon Mallal 2, 4 , Joseph A Conrad 7 , Spyros A Kalams 4 , Silvana Gaudieri 1, 2, 4
Affiliation
|
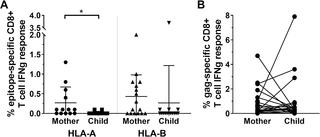
Human immunodeficiency virus (HIV) can adapt to an individual's T cell immune response via genomic mutations that affect antigen recognition and impact disease outcome. These viral adaptations are specific to the host's human leucocyte antigen (HLA) alleles, as these molecules determine which peptides are presented to T cells. As HLA molecules are highly polymorphic at the population level, horizontal transmission events are most commonly between HLA-mismatched donor/recipient pairs, representing new immune selection environments for the transmitted virus. In this study, we utilised a deep sequencing approach to determine the HIV quasispecies in 26 mother-to-child transmission pairs where the potential for founder viruses to be pre-adapted is high due to the pairs being haplo-identical at HLA loci. This scenario allowed the assessment of specific HIV adaptations following transmission in either a non-selective immune environment, due to recipient HLA mismatched to original selecting HLA, or a selective immune environment, mediated by matched donor/recipient HLA. We show that the pattern of reversion or fixation of HIV adaptations following transmission provides insight into the replicative cost, and likely compensatory networks, associated with specific adaptations in vivo. Furthermore, although transmitted viruses were commonly heavily pre-adapted to the child's HLA genotype, we found evidence of de novo post-transmission adaptation, representing new epitopes targeted by the child's T cell response. High-resolution analysis of HIV adaptation is relevant when considering vaccine and cure strategies for individuals exposed to adapted viruses via transmission or reactivated from reservoirs.
中文翻译:

垂直传播后对HIV适应的深度序列分析揭示了免疫压力对HIV进化的影响。
人类免疫缺陷病毒(HIV)可以通过影响抗原识别和影响疾病结果的基因组突变来适应个体的T细胞免疫反应。这些病毒适应性特异于宿主的人白细胞抗原(HLA)等位基因,因为这些分子决定了将哪些肽呈递给T细胞。由于HLA分子在种群水平上具有高度多态性,因此水平传播事件最常见于HLA不匹配的供体/受体对之间,代表了传播病毒的新免疫选择环境。在这项研究中,我们采用了深度测序方法来确定26对母婴传播对中的HIV准种,其中由于HLA基因座上的单倍同源性,对创始人病毒的潜在适应性很高。由于受体HLA与原始选择的HLA不匹配,或者由于匹配的供体/受体HLA介导的选择性免疫环境,这种情况允许评估在非选择性免疫环境中传播后特定HIV的适应性。我们表明,传播后HIV适应症的逆转或固定模式可提供与体内特定适应症相关的复制成本和可能的补偿网络的洞察力。此外,尽管传播的病毒通常高度适应儿童的HLA基因型,但我们发现了从头传播后适应的证据,代表了儿童T细胞反应靶向的新抗原决定簇。
更新日期:2019-12-11
中文翻译:

垂直传播后对HIV适应的深度序列分析揭示了免疫压力对HIV进化的影响。
人类免疫缺陷病毒(HIV)可以通过影响抗原识别和影响疾病结果的基因组突变来适应个体的T细胞免疫反应。这些病毒适应性特异于宿主的人白细胞抗原(HLA)等位基因,因为这些分子决定了将哪些肽呈递给T细胞。由于HLA分子在种群水平上具有高度多态性,因此水平传播事件最常见于HLA不匹配的供体/受体对之间,代表了传播病毒的新免疫选择环境。在这项研究中,我们采用了深度测序方法来确定26对母婴传播对中的HIV准种,其中由于HLA基因座上的单倍同源性,对创始人病毒的潜在适应性很高。由于受体HLA与原始选择的HLA不匹配,或者由于匹配的供体/受体HLA介导的选择性免疫环境,这种情况允许评估在非选择性免疫环境中传播后特定HIV的适应性。我们表明,传播后HIV适应症的逆转或固定模式可提供与体内特定适应症相关的复制成本和可能的补偿网络的洞察力。此外,尽管传播的病毒通常高度适应儿童的HLA基因型,但我们发现了从头传播后适应的证据,代表了儿童T细胞反应靶向的新抗原决定簇。











































 京公网安备 11010802027423号
京公网安备 11010802027423号