当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
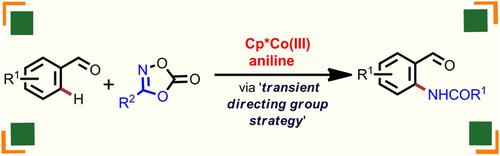
Cp*Co(III)‐Catalyzed o‐Amidation of Benzaldehydes with Dioxazolones Using Transient Directing Group Strategy
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-08 , DOI: 10.1002/adsc.201901267 Bhuttu Khan 1 , Vikas Dwivedi 1 , Basker Sundararaju 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-01-08 , DOI: 10.1002/adsc.201901267 Bhuttu Khan 1 , Vikas Dwivedi 1 , Basker Sundararaju 1
Affiliation
|
Transition metal‐catalyzed ortho‐selective C(sp2)−H amidation of weakly coordinating aldehydes remains limited to precious metals such as Ir, Rh, Ru, etc. Herein, we put forward a novel report on ortho‐amidation of benzaldehydes employing user‐friendly dioxazolones under cost‐effective and air‐stable Cp*Co(III) catalysis. This catalytic transformation involves transient directing group strategy to enhance the ligating capability of weakly chelating aldehydes.
中文翻译:

Cp * Co(III)瞬态定向基团策略催化苯甲醛与二恶唑酮的邻氨基酰胺化
过渡金属催化的邻选择性C(SP 2)-H的弱配位醛遗体局限于贵金属如铱,铑,钌等。这里酰胺化,提出了一种新颖的报告邻采用用户苯甲醛的酰胺化经济高效且空气稳定的Cp * Co(III)催化下的友好型二恶唑酮。这种催化转化涉及瞬态导向基团策略,以增强弱螯合醛的连接能力。
更新日期:2020-01-08
中文翻译:

Cp * Co(III)瞬态定向基团策略催化苯甲醛与二恶唑酮的邻氨基酰胺化
过渡金属催化的邻选择性C(SP 2)-H的弱配位醛遗体局限于贵金属如铱,铑,钌等。这里酰胺化,提出了一种新颖的报告邻采用用户苯甲醛的酰胺化经济高效且空气稳定的Cp * Co(III)催化下的友好型二恶唑酮。这种催化转化涉及瞬态导向基团策略,以增强弱螯合醛的连接能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号