当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Heck Arylation of Acyclic Alkenol Aryl Ethers: Synthetic Applications and DFT Investigation of the Stereoselectivity
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-12-23 , DOI: 10.1002/adsc.201901471 Ellen Christine Polo 1 , Martí Fernández Wang 1 , Ricardo Almir Angnes 2 , Ataualpa A. C. Braga 2 , Carlos Roque Duarte Correia 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-12-23 , DOI: 10.1002/adsc.201901471 Ellen Christine Polo 1 , Martí Fernández Wang 1 , Ricardo Almir Angnes 2 , Ataualpa A. C. Braga 2 , Carlos Roque Duarte Correia 1
Affiliation
|
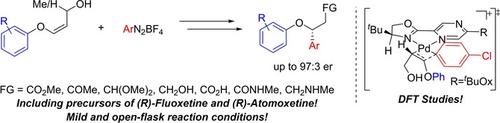
Herein we report the enantioselective Heck‐Matsuda arylation of acyclic E and Z‐alkenyl aryl ethers. The reactions were carried out under mild conditions affording the enantioenriched benzyl ethers in a regioselective manner, moderate to good yields (up to 73%), and in good to excellent enantiomeric ratios (up to 97:3). The enantioselective Heck‐Matsuda arylation has shown a broad scope (25 examples), and some key Heck‐Matsuda adducts were further converted into more complex and valuable scaffolds including their synthetic application in the synthesis of (R)‐Fluoxetine, (R)‐Atomoxetine, and in the synthesis of an enantioenriched benzo[c]chromene. Finally, in silico mechanistic investigations into the reaction's enantioselectivity were performed using density functional theory.
中文翻译:

无环烯醇芳醚的对映选择性颈缩丙烯酸化:立体选择性的合成应用和DFT研究
在本文中,我们报告了无环E和Z链烯基芳基醚的对映选择性Heck-Matsuda芳基化。反应在温和的条件下进行,以区域选择性的方式得到对映体富集的苄基醚,中等至良好的收率(高达73%),以及良好至优异的对映体比率(高达97:3)。对映体选择性的Heck-松田芳基化已经显示出宽范围(25个实施例),并且一些关键的Heck-松田加合物进一步转化成更复杂的和有价值的支架包括在(合成及其合成应用- [R)-Fluoxetine,(- [R )-托莫西汀,以及在对映体富集的苯并[ c ]色烯的合成中。最后,在计算机上 使用密度泛函理论对反应的对映选择性进行了机理研究。
更新日期:2019-12-23
中文翻译:

无环烯醇芳醚的对映选择性颈缩丙烯酸化:立体选择性的合成应用和DFT研究
在本文中,我们报告了无环E和Z链烯基芳基醚的对映选择性Heck-Matsuda芳基化。反应在温和的条件下进行,以区域选择性的方式得到对映体富集的苄基醚,中等至良好的收率(高达73%),以及良好至优异的对映体比率(高达97:3)。对映体选择性的Heck-松田芳基化已经显示出宽范围(25个实施例),并且一些关键的Heck-松田加合物进一步转化成更复杂的和有价值的支架包括在(合成及其合成应用- [R)-Fluoxetine,(- [R )-托莫西汀,以及在对映体富集的苯并[ c ]色烯的合成中。最后,在计算机上 使用密度泛函理论对反应的对映选择性进行了机理研究。









































 京公网安备 11010802027423号
京公网安备 11010802027423号