当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Constrained and "Inverted" [3+3] Salphen Macrocycle with an ortho-Phenylethynyl Substitution Pattern.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-01-23 , DOI: 10.1002/chem.201904763 Maxence Urbani 1, 2 , Tomas Torres 1, 2, 3
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-01-23 , DOI: 10.1002/chem.201904763 Maxence Urbani 1, 2 , Tomas Torres 1, 2, 3
Affiliation
|
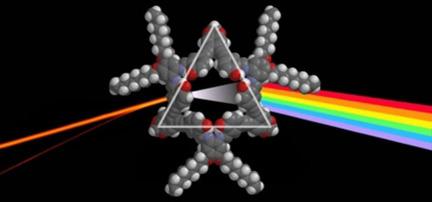
A [3+3] Schiff-base salphen macrocycle (7 a) was synthesized by imine condensation between ortho-phenylenediamine and ortho-phenylethynyl-bridged bis(5-salicylaldehyde) precursors. The triangular-shaped macrocycle 7 a has a nonclassical (or "inverted") design in which the N2 O2 coordination pockets are located at the sides instead of the corners. Compound 7 a could be synthesized in a reasonably good yield (64 %) considering the steric constraints imposed by the ortho substitution pattern. Subsequent zinc metalation afforded the corresponding Zn metallomacrocycle 7 b. Spectroscopic experiments evidenced weak (7 a) to strong (7 b) self-aggregation behavior in solution. Their ability to self-organize at the supramolecular level was further studied in the solid state by AFM and TEM, which revealed the formation of large bundles of fibers with lengths of several micrometers and widths of nanometers.
中文翻译:

具有邻苯乙炔基取代模式的受约束且“反转”的[3 + 3] Salphen大环。
通过邻苯二胺和邻苯乙炔基桥联的双(5-水杨醛)前体之间的亚胺缩合合成了[3 + 3]席夫碱赛尔芬大环化合物(7a)。三角形大环7a具有非经典(或“倒置”)设计,其中N 2 O 2配位袋位于侧面而不是拐角处。考虑到邻位取代模式所施加的空间限制,化合物7a的合成产率可以相当高(64%)。随后的锌金属化得到相应的Zn金属大环7b。光谱实验证明溶液中的自聚集行为从弱(7 a)到强(7 b)。通过固态原子力显微镜(AFM)和透射电镜(TEM)进一步研究了它们在超分子水平上的自组织能力,
更新日期:2020-01-23
中文翻译:

具有邻苯乙炔基取代模式的受约束且“反转”的[3 + 3] Salphen大环。
通过邻苯二胺和邻苯乙炔基桥联的双(5-水杨醛)前体之间的亚胺缩合合成了[3 + 3]席夫碱赛尔芬大环化合物(7a)。三角形大环7a具有非经典(或“倒置”)设计,其中N 2 O 2配位袋位于侧面而不是拐角处。考虑到邻位取代模式所施加的空间限制,化合物7a的合成产率可以相当高(64%)。随后的锌金属化得到相应的Zn金属大环7b。光谱实验证明溶液中的自聚集行为从弱(7 a)到强(7 b)。通过固态原子力显微镜(AFM)和透射电镜(TEM)进一步研究了它们在超分子水平上的自组织能力,











































 京公网安备 11010802027423号
京公网安备 11010802027423号