当前位置:
X-MOL 学术
›
DNA Repair
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Macromolecular crowding induces compaction and DNA binding in the disordered N-terminal domain of hUNG2.
DNA Repair ( IF 3.0 ) Pub Date : 2019-12-10 , DOI: 10.1016/j.dnarep.2019.102764 Gaddiel Rodriguez 1 , Benjamin Orris 1 , Ananya Majumdar 2 , Shridhar Bhat 1 , James T Stivers 1
DNA Repair ( IF 3.0 ) Pub Date : 2019-12-10 , DOI: 10.1016/j.dnarep.2019.102764 Gaddiel Rodriguez 1 , Benjamin Orris 1 , Ananya Majumdar 2 , Shridhar Bhat 1 , James T Stivers 1
Affiliation
|
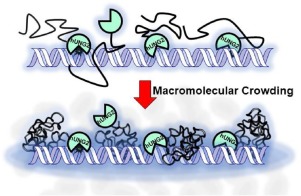
Many human DNA repair proteins have disordered domains at their N- or C-termini with poorly defined biological functions. We recently reported that the partially structured N-terminal domain (NTD) of human uracil DNA glycosylase 2 (hUNG2), functions to enhance DNA translocation in crowded environments and also targets the enzyme to single-stranded/double-stranded DNA junctions. To understand the structural basis for these effects we now report high-resolution heteronuclear NMR studies of the isolated NTD in the presence and absence of an inert macromolecular crowding agent (PEG8K). Compared to dilute buffer, we find that crowding reduces the degrees of freedom for the structural ensemble, increases the order of a PCNA binding motif and dramatically promotes binding of the NTD for DNA through a conformational selection mechanism. These findings shed new light on the function of this disordered domain in the context of the crowded nuclear environment.
中文翻译:

大分子拥挤诱导hUNG2的无序N末端域中的压缩和DNA结合。
许多人类DNA修复蛋白在其N末端或C末端具有混乱的结构域,生物学功能定义不清。我们最近报道了人尿嘧啶DNA糖基化酶2(hUNG2)的部分结构化的N末端结构域(NTD),其功能是在拥挤的环境中增强DNA的易位性,并将该酶靶向到单链/双链DNA连接处。为了了解这些效应的结构基础,我们现在报告在存在和不存在惰性大分子拥挤剂(PEG8K)的情况下,对分离出的NTD进行高分辨率异核NMR研究。与稀缓冲液相比,我们发现拥挤降低了结构整体的自由度,增加了PCNA结合基序的顺序,并通过构象选择机制显着促进了NTD与DNA的结合。
更新日期:2019-12-11
中文翻译:

大分子拥挤诱导hUNG2的无序N末端域中的压缩和DNA结合。
许多人类DNA修复蛋白在其N末端或C末端具有混乱的结构域,生物学功能定义不清。我们最近报道了人尿嘧啶DNA糖基化酶2(hUNG2)的部分结构化的N末端结构域(NTD),其功能是在拥挤的环境中增强DNA的易位性,并将该酶靶向到单链/双链DNA连接处。为了了解这些效应的结构基础,我们现在报告在存在和不存在惰性大分子拥挤剂(PEG8K)的情况下,对分离出的NTD进行高分辨率异核NMR研究。与稀缓冲液相比,我们发现拥挤降低了结构整体的自由度,增加了PCNA结合基序的顺序,并通过构象选择机制显着促进了NTD与DNA的结合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号